A laboratory procedure calls for making 400.0 mL of a 1.1 M NaNO3 solution. What mass of NaNO3 (in g) is needed?
Ch.5 - Introduction to Solutions and Aqueous Solutions

Chapter 5, Problem 30
If 3.5 L of a 4.8 M SrCl2 solution is diluted to 45 L, what is the molarity of the diluted solution?
 Verified step by step guidance
Verified step by step guidance1
Identify the initial conditions: the initial volume \( V_1 = 3.5 \) L and the initial molarity \( M_1 = 4.8 \) M.
Identify the final volume after dilution: \( V_2 = 45 \) L.
Use the dilution formula: \( M_1 \times V_1 = M_2 \times V_2 \), where \( M_2 \) is the molarity of the diluted solution.
Rearrange the formula to solve for \( M_2 \): \( M_2 = \frac{M_1 \times V_1}{V_2} \).
Substitute the known values into the equation: \( M_2 = \frac{4.8 \times 3.5}{45} \) to find the molarity of the diluted solution.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Molarity
Molarity (M) is a measure of concentration defined as the number of moles of solute per liter of solution. It is expressed in moles per liter (mol/L) and is crucial for understanding how much solute is present in a given volume of solution. Molarity is commonly used in chemistry to prepare solutions and perform stoichiometric calculations.
Recommended video:
Guided course

Molarity
Dilution
Dilution is the process of reducing the concentration of a solute in a solution, typically by adding more solvent. The dilution equation, M1V1 = M2V2, relates the initial and final molarity (M) and volume (V) of the solution. This concept is essential for calculating the new concentration after a solution has been diluted.
Recommended video:
Guided course
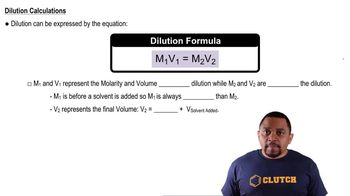
Dilution Equation
Conservation of Moles
The conservation of moles principle states that the number of moles of solute remains constant before and after dilution, assuming no solute is added or removed. This principle underlies the dilution equation and allows for the calculation of the final concentration by equating the moles of solute in the initial and diluted solutions.
Recommended video:
Guided course
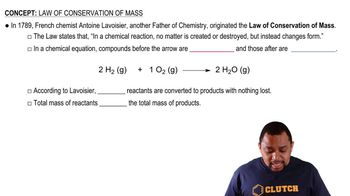
Law of Conservation of Mass
Related Practice
Textbook Question
Textbook Question
A chemist wants to make 5.5 L of a 0.300 M CaCl2 solution. What mass of CaCl2 (in g) should the chemist use?
Textbook Question
If 123 mL of a 1.1 M glucose solution is diluted to 500.0 mL, what is the molarity of the diluted solution?
1
views
Textbook Question
To what volume should you dilute 50.0 mL of a 12 M stock HNO3 solution to obtain a 0.100 M HNO3 solution?
Textbook Question
What is the minimum amount of 6.0 M H2SO4 necessary to produce 25.0 g of H2(g) according to the reaction between aluminum and sulfuric acid? 2 Al(s) + 3 H2SO4(aq) → Al2(SO4)3(aq) + 3 H2(g)
1
views
Textbook Question
Consider the reaction: Li2S(aq) + Co(NO3)2(aq) → 2 LiNO3(aq) + CoS(s) What volume of 0.150 M Li2S solution is required to completely react with 125 mL of 0.150 M Co(NO3)2?
