Many computer chips are manufactured from silicon, which occurs in nature as SiO2. When SiO2 is heated to melting, it reacts with solid carbon to form liquid silicon and carbon monoxide gas. In an industrial preparation of silicon, 155.8 kg of SiO2 reacts with 78.3 kg of carbon to produce 66.1 kg of silicon. Determine the percent yield for the reaction.
Ch.4 - Chemical Reactions and Chemical Quantities

Chapter 4, Problem 55
Write the balanced chemical equation for the reaction of solid lithium with liquid water.
 Verified step by step guidance
Verified step by step guidance1
Identify the reactants and products in the reaction: solid lithium (Li) and liquid water (H2O) react to form lithium hydroxide (LiOH) and hydrogen gas (H2).
Write the unbalanced chemical equation: Li(s) + H2O(l) -> LiOH(aq) + H2(g).
Balance the lithium atoms: Since there is one lithium atom on both sides, lithium is already balanced.
Balance the hydrogen atoms: There are two hydrogen atoms in water and two in hydrogen gas, so the hydrogen atoms are balanced. However, the hydroxide ion (OH-) in LiOH also contains a hydrogen atom, so adjust the coefficients to balance all hydrogen atoms.
Balance the oxygen atoms: Ensure that the number of oxygen atoms is the same on both sides of the equation by adjusting the coefficients as necessary.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Balancing Chemical Equations
Balancing chemical equations involves ensuring that the number of atoms of each element is the same on both sides of the equation. This is based on the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. To balance an equation, coefficients are adjusted in front of the chemical formulas.
Recommended video:
Guided course

Balancing Chemical Equations
Reactivity of Alkali Metals
Alkali metals, such as lithium, are highly reactive, especially with water. When they react with water, they typically produce a hydroxide and hydrogen gas. This reaction is exothermic, meaning it releases heat, and can be vigorous, leading to the potential for flames or explosions if not handled properly.
Recommended video:
Guided course

Transition Metals
Products of Metal-Water Reactions
The reaction between a metal and water generally produces a metal hydroxide and hydrogen gas. In the case of lithium, the reaction yields lithium hydroxide (LiOH) and hydrogen (H2). Understanding the products formed in such reactions is crucial for predicting the outcomes of chemical processes involving metals and water.
Recommended video:
Guided course
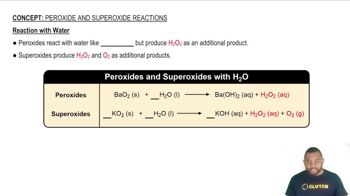
Reaction with Water
Related Practice
Textbook Question
1
views
1
rank
Textbook Question
Write the balanced chemical equation for the reaction of solid strontium with iodine gas.
Textbook Question
Write the balanced chemical equation for the reaction of solid potassium with liquid water.
Textbook Question
Write the balanced equation for the reaction of hydrogen gas with bromine gas.
1
views
Textbook Question
The combustion of gasoline produces carbon dioxide and water. Assume gasoline to be pure octane (C8H18) and calculate the mass (in kg) of carbon dioxide that is added to the atmosphere per 1.0 kg of octane burned. (Hint: Begin by writing a balanced equation for the combustion reaction.)
1
views
