Textbook Question
How many molecules are in each sample? b. 389 g CBr4
1
views

 Verified step by step guidance
Verified step by step guidance


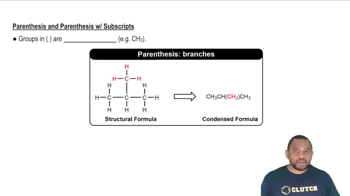
How many molecules are in each sample? b. 389 g CBr4
How many atoms are specified by each of these prefixes: mono-, di-, tri-, tetra-, penta-, hexa-?
Determine the number of each type of atom in each formula. b. CuSO4 d. Mg(HCO3)2
Determine the number of each type of atom in each formula. c. Al(NO3)3
Write a chemical formula for each molecular model. (See Appendix IIA for color codes.) (a)