Calculate the empirical formula for each natural flavor based on its elemental mass percent composition. a. methyl butyrate (component of apple taste and smell): C 58.80%, H 9.87%, O 31.33%

Calculate the empirical formula for each stimulant based on its elemental mass percent composition.
a. nicotine (found in tobacco leaves): C 74.03%, H 8.70%, N 17.27%
b. caffeine (found in coffee beans): C 49.48%, H 5.19%, N 28.85%, O 16.48%
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
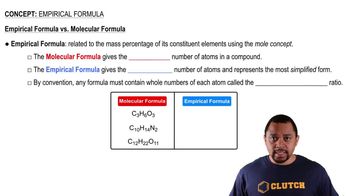
Key Concepts
Empirical Formula
Mass Percent Composition

Mole Concept

Write a ratio showing the relationship between the molar amounts of each element for each compound. (See Appendix IIA for color codes.) (b)
A chemist decomposes samples of several compounds; the masses of their constituent elements are listed. Calculate the empirical formula for each compound. b. 2.677 g Ba, 3.115 g Br
A chemist decomposes samples of several compounds; the masses of their constituent elements are listed. Calculate the empirical formula for each compound. c. 2.128 g Be, 7.557 g S, 15.107 g O
Calculate the empirical formula for each natural flavor based on its elemental mass percent composition. b. vanillin (responsible for the taste and smell of vanilla): C 63.15%, H 5.30%, O 31.55%
The elemental mass percent composition of ibuprofen (a nonsteroidal anti-inflammatory drug [NSAID]) is 75.69% C, 8.80% H, and 15.51% O. Determine the empirical formula of ibuprofen.
