Predict a likely mode of decay for each unstable nuclide. c. Fr-202
Ch.21 - Radioactivity & Nuclear Chemistry

Chapter 21, Problem 42a
Predict a likely mode of decay for each unstable nuclide. a. Sb-132 b. Te-139 d. Ba-123
 Verified step by step guidance
Verified step by step guidance1
Identify the atomic number of the element. Antimony (Sb) has an atomic number of 51.
Determine the neutron number by subtracting the atomic number from the mass number: Neutron number = 132 - 51.
Compare the neutron-to-proton (n/p) ratio to the stable n/p ratio for elements around antimony. A high n/p ratio suggests beta decay, while a low n/p ratio suggests positron emission or electron capture.
Consider the position of the nuclide on the chart of nuclides. Nuclides above the band of stability typically undergo beta decay to decrease the neutron number.
Conclude the likely mode of decay based on the n/p ratio and the nuclide's position relative to the band of stability.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Nuclear Decay
Nuclear decay refers to the process by which an unstable atomic nucleus loses energy by emitting radiation. This can occur through various modes, including alpha decay, beta decay, and gamma decay, each characterized by the type of particle or energy released. Understanding the stability of nuclides and the forces at play within the nucleus is essential for predicting the mode of decay.
Recommended video:
Guided course
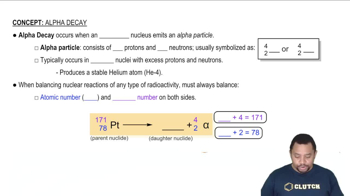
Alpha Decay
Beta Decay
Beta decay is a specific type of radioactive decay in which a neutron in the nucleus is transformed into a proton, emitting a beta particle (an electron or positron) and an antineutrino or neutrino. This process increases the atomic number of the element by one while keeping the mass number unchanged. It is common in nuclides that are neutron-rich, making it a likely decay mode for certain isotopes.
Recommended video:
Guided course

Beta Decay
Isotopes and Stability
Isotopes are variants of a chemical element that have the same number of protons but different numbers of neutrons, leading to different mass numbers. The stability of an isotope depends on the ratio of neutrons to protons; an imbalance can lead to instability and radioactive decay. Understanding the specific isotope in question, such as Sb-132, is crucial for predicting its decay mode based on its nuclear structure.
Recommended video:
Guided course

Isotopes
Related Practice
Textbook Question
Textbook Question
The first six elements of the first transition series have the following number of stable isotopes:
Element Number of Stable Isotopes
Sc 1
Ti 5
V 1
Cr 3
Mn 1
Fe 4
Explain why Sc, V, and Mn each have only one stable isotope while the other elements have several.
1
views
Textbook Question
Predict a likely mode of decay for each unstable nuclide. a. Mo-109
1
views
Textbook Question
Predict a likely mode of decay for each unstable nuclide. b. Ru-90 c. P-27 d. Sn-100
Textbook Question
Which nuclide in each pair would you expect to have the longer half-life? a. Cs-149 or Cs-139 b. Fe-45 or Fe-52
Textbook Question
One of the nuclides in spent nuclear fuel is U-235, an alpha emitter with a half-life of 703 million years. How long will it take for the amount of U-235 to reach 10.0% of its initial amount?
