For each reaction, calculate ΔH°rxn, ΔS°rxn, and ΔG°rxn at 25 °C and state whether or not the reaction is spontaneous. If the reaction is not spontaneous, would a change in temperature make it spontaneous? If so, should the temperature be raised or lowered from 25 °C? a. 2 CH4(g) → C2H6(g) + H2(g)
Ch.19 - Free Energy & Thermodynamics

Chapter 19, Problem 62d
For each reaction, calculate ΔH°rxn, ΔS°rxn, and ΔG°rxn at 25 °C and state whether or not the reaction is spontaneous. If the reaction is not spontaneous, would a change in temperature make it spontaneous? If so, should the temperature be raised or lowered from 25 °C? d. 2 KClO3(s) → 2 KCl(s) + 3 O2(g)
 Verified step by step guidance
Verified step by step guidance1
Identify the standard enthalpy change (\( \Delta H^\circ \)) for the reaction by using standard enthalpies of formation (\( \Delta H_f^\circ \)) for each compound involved. Use the formula: \( \Delta H^\circ_{\text{rxn}} = \sum \Delta H_f^\circ (\text{products}) - \sum \Delta H_f^\circ (\text{reactants}) \).
Determine the standard entropy change (\( \Delta S^\circ \)) for the reaction using standard molar entropies (\( S^\circ \)) for each compound. Use the formula: \( \Delta S^\circ_{\text{rxn}} = \sum S^\circ (\text{products}) - \sum S^\circ (\text{reactants}) \).
Calculate the standard Gibbs free energy change (\( \Delta G^\circ \)) for the reaction at 25 °C using the equation: \( \Delta G^\circ_{\text{rxn}} = \Delta H^\circ_{\text{rxn}} - T \Delta S^\circ_{\text{rxn}} \), where \( T \) is the temperature in Kelvin (298 K).
Assess the spontaneity of the reaction by examining the sign of \( \Delta G^\circ_{\text{rxn}} \). If \( \Delta G^\circ_{\text{rxn}} < 0 \), the reaction is spontaneous at 25 °C. If \( \Delta G^\circ_{\text{rxn}} > 0 \), the reaction is non-spontaneous at 25 °C.
If the reaction is non-spontaneous, consider how temperature affects spontaneity. If \( \Delta H^\circ > 0 \) and \( \Delta S^\circ > 0 \), increasing temperature may make the reaction spontaneous. If \( \Delta H^\circ < 0 \) and \( \Delta S^\circ < 0 \), decreasing temperature may make the reaction spontaneous.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
9mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Thermodynamic Functions (ΔH, ΔS, ΔG)
Thermodynamic functions such as enthalpy change (ΔH), entropy change (ΔS), and Gibbs free energy change (ΔG) are essential for understanding chemical reactions. ΔH indicates the heat absorbed or released during a reaction, ΔS measures the disorder or randomness of the system, and ΔG determines the spontaneity of the reaction. A negative ΔG indicates a spontaneous reaction, while a positive ΔG suggests non-spontaneity.
Recommended video:
Guided course

First Law of Thermodynamics
Spontaneity of Reactions
The spontaneity of a reaction is determined by the sign of ΔG. A reaction is spontaneous at a given temperature if ΔG is negative, meaning it can occur without external energy. The relationship between ΔH, ΔS, and temperature is described by the equation ΔG = ΔH - TΔS, indicating that changes in temperature can influence spontaneity, especially for reactions with positive ΔH and ΔS.
Recommended video:
Guided course
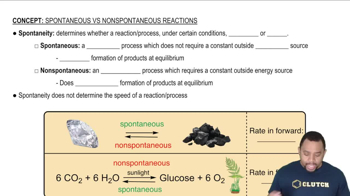
Spontaneity of Processes
Temperature's Effect on Spontaneity
Temperature plays a crucial role in determining the spontaneity of a reaction, particularly when ΔH and ΔS have opposing signs. For reactions where ΔH is positive and ΔS is also positive, increasing the temperature can make ΔG negative, thus making the reaction spontaneous. Conversely, if ΔH is negative and ΔS is negative, lowering the temperature may be necessary to achieve spontaneity.
Recommended video:
Guided course
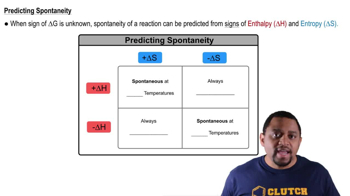
Spontaneity and Temperature
Related Practice
Textbook Question
Textbook Question
For each reaction, calculate ΔH°rxn, ΔS°rxn, and ΔG°rxn at 25 °C and state whether or not the reaction is spontaneous. If the reaction is not spontaneous, would a change in temperature make it spontaneous? If so, should the temperature be raised or lowered from 25 °C? c. N2(g) + O2(g) → 2 NO(g)
Textbook Question
Use standard free energies of formation to calculate ΔG° at 25 °C for each reaction in Problem 61. How do the values of ΔG° calculated this way compare to those calculated from ΔH° and ΔS°? Which of the two methods could be used to determine how ΔG° changes with temperature?
1
views
