Consider the reaction: I2(g) + Cl2(g) ⇌ 2 ICl(g) Kp = 81.9 at 25 °C Calculate ΔGrxn for the reaction at 25 °C under each of the following conditions: a. standard conditions
Ch.19 - Free Energy & Thermodynamics

Chapter 19, Problem 77
Is the value of the equilibrium constant at 525 K for each reaction in Problem 73 estimated correctly?
 Verified step by step guidance
Verified step by step guidance1
insert step 1> Identify the reactions from Problem 73 that need to be evaluated for their equilibrium constants at 525 K.
insert step 2> Review the method used to estimate the equilibrium constant for each reaction. This could involve calculations using the Van't Hoff equation or other thermodynamic data.
insert step 3> Check if the temperature dependence of the equilibrium constant was considered correctly. This often involves using the Van't Hoff equation: \( \ln \frac{K_2}{K_1} = -\frac{\Delta H^\circ}{R} \left( \frac{1}{T_2} - \frac{1}{T_1} \right) \), where \( \Delta H^\circ \) is the standard enthalpy change, \( R \) is the gas constant, and \( T_1 \) and \( T_2 \) are the initial and final temperatures, respectively.
insert step 4> Verify if the correct values for \( \Delta H^\circ \) and \( K_1 \) (the equilibrium constant at the initial temperature) were used in the calculations.
insert step 5> Compare the estimated equilibrium constants with any available experimental data or literature values to assess the accuracy of the estimates.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Equilibrium Constant (K)
The equilibrium constant (K) is a numerical value that expresses the ratio of the concentrations of products to reactants at equilibrium for a given reaction at a specific temperature. It provides insight into the extent of a reaction and whether it favors products or reactants. A larger K value indicates a greater concentration of products at equilibrium, while a smaller K suggests a predominance of reactants.
Recommended video:
Guided course

Equilibrium Constant K
Temperature Dependence of K
The value of the equilibrium constant is temperature-dependent, meaning it can change with variations in temperature. According to Le Chatelier's principle, if a reaction is exothermic, increasing the temperature will decrease the value of K, while for endothermic reactions, increasing temperature will increase K. Understanding this relationship is crucial for accurately estimating K at different temperatures.
Recommended video:
Guided course
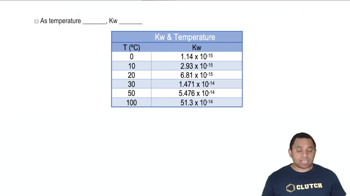
Kw Temperature Dependence
Reaction Quotient (Q)
The reaction quotient (Q) is a measure of the relative concentrations of products and reactants at any point in time during a reaction. It is calculated using the same formula as K but with concentrations that are not necessarily at equilibrium. Comparing Q to K helps predict the direction in which a reaction will proceed to reach equilibrium, which is essential for evaluating the correctness of K values in specific conditions.
Recommended video:
Guided course

Reaction Quotient Q
Related Practice
Textbook Question
Textbook Question
Consider the reaction: I2(g) + Cl2(g) ⇌ 2 ICl(g) Kp = 81.9 at 25 °C Calculate ΔGrxn for the reaction at 25 °C under each of the following conditions: b. at equilibrium
Textbook Question
Consider the reaction: I2(g) + Cl2(g) ⇌ 2 ICl(g) Kp = 81.9 at 25 °C Calculate ΔGrxn for the reaction at 25 °C under each of the following conditions: c. PICl = 2.55 atm; PI2 = 0.325 atm; PCl2 = 0.221 atm
1
views
Textbook Question
Consider the reaction: 2 NO(g) + O2(g) ⇌ 2 NO2(g) The following data show the equilibrium constant for this reaction measured at several different temperatures. Use the data to find ΔH°rxn and ΔS°rxn for the reaction.
Textbook Question
A reaction has an equilibrium constant of 8.5⨉103 at 298 K. At 755 K, the equilibrium constant is 0.65. Find ΔH°rxn for the reaction.
