Consider the sublimation of iodine at 25.0 °C : I2(s) → I2(g) c. Explain why iodine spontaneously sublimes in open air at 25.0 °C
Ch.19 - Free Energy & Thermodynamics

Chapter 19, Problem 70c
Consider the evaporation of methanol at 25.0 °C : CH3OH(l) → CH3OH(g) c. Explain why methanol spontaneously evaporates in open air at 25.0 °C
 Verified step by step guidance
Verified step by step guidance1
insert step 1: Understand the concept of spontaneity in thermodynamics. A process is spontaneous if it occurs without needing to be driven by an external force.
insert step 2: Consider the Gibbs free energy change (ΔG) for the evaporation process. A process is spontaneous if ΔG is negative.
insert step 3: Use the equation ΔG = ΔH - TΔS, where ΔH is the change in enthalpy, T is the temperature in Kelvin, and ΔS is the change in entropy.
insert step 4: Recognize that evaporation involves a phase change from liquid to gas, which increases the disorder or entropy (ΔS > 0) of the system.
insert step 5: Even if the enthalpy change (ΔH) is positive (endothermic), the increase in entropy (ΔS) can make the TΔS term large enough to result in a negative ΔG, making the process spontaneous.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Evaporation
Evaporation is the process by which molecules at the surface of a liquid gain enough energy to enter the gas phase. This occurs when some molecules have sufficient kinetic energy to overcome intermolecular forces, allowing them to escape into the air. The rate of evaporation increases with temperature, as higher temperatures provide more molecules with the energy needed to evaporate.
Recommended video:
Guided course
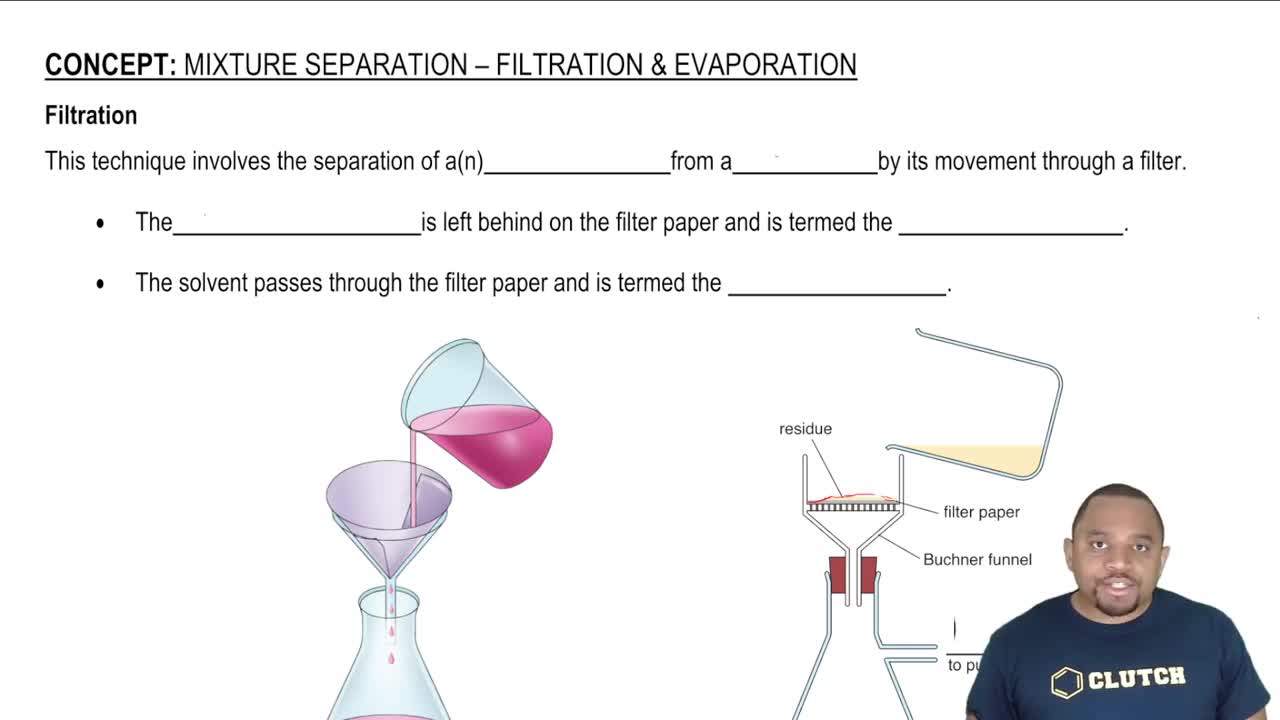
Filtration and Evaporation
Vapor Pressure
Vapor pressure is the pressure exerted by a vapor in equilibrium with its liquid at a given temperature. For methanol at 25.0 °C, the vapor pressure is significant enough that some molecules will escape into the air, leading to spontaneous evaporation. When the vapor pressure exceeds the atmospheric pressure, evaporation occurs more readily.
Recommended video:
Guided course

Raoult's Law and Vapor Pressure
Thermodynamics and Spontaneity
Thermodynamics helps explain the spontaneity of processes based on changes in energy and entropy. The evaporation of methanol is spontaneous at 25.0 °C because it increases the entropy of the system; as liquid methanol transitions to gas, the disorder increases, favoring the process. This aligns with the second law of thermodynamics, which states that spontaneous processes tend to increase the overall entropy of the universe.
Recommended video:
Guided course

Spontaneity of Processes
Related Practice
Textbook Question
Textbook Question
Consider the evaporation of methanol at 25.0 °C : CH3OH(l) → CH3OH(g) a. Find ΔG°r at 25.0 °C.
1
views
Textbook Question
Consider the evaporation of methanol at 25.0 °C : CH3OH(l) → CH3OH(g) b. Find ΔGr at 25.0 °C under the following nonstandard conditions: i. PCH3OH = 150.0 mmHg ii. PCH3OH = 100.0 mmHg iii. PCH3OH = 10.0 mmHg
Textbook Question
Consider the reaction: CO2(g) + CCl4(g) ⇌ 2 COCl2(g) Calculate ΔG for this reaction at 25 °C under the following conditions: i. PCO2 = 0.112 atm ii. PCCl4 = 0.174 atm iii. PCOCl2 = 0.744 atm
Textbook Question
Use data from Appendix IIB to calculate the equilibrium constants at 25 °C for each reaction. a. 2 CO(g) + O2(g) ⇌ 2 CO2(g)
Textbook Question
Use data from Appendix IIB to calculate the equilibrium constants at 25 °C for each reaction. b. 2 H2S(g) ⇌ 2 H2(g) + S2(g)
