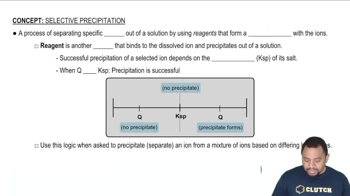
Textbook Question
A solution containing sodium fluoride is mixed with one containing calcium nitrate to form a solution that is 0.015 M in NaF and 0.010 M in Ca(NO3)2. Does a precipitate form in the mixed solution? If so, identify the precipitate.

 Verified step by step guidance
Verified step by step guidance
A solution containing sodium fluoride is mixed with one containing calcium nitrate to form a solution that is 0.015 M in NaF and 0.010 M in Ca(NO3)2. Does a precipitate form in the mixed solution? If so, identify the precipitate.
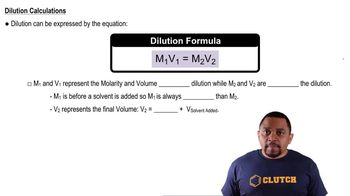
Predict whether a precipitate will form if you mix 75.0 mL of a NaOH solution with pOH = 2.58 with 125.0 mL of a 0.018 M MgCl2 solution. Identify the precipitate, if any.
Determine the minimum concentration of the precipitating agent on the right to cause precipitation of the cation from the solution on the left. a. 0.035 M Ba(NO3)2; NaF
Determine the minimum concentration of the precipitating agent on the right to cause precipitation of the cation from the solution on the left. b. 0.085 M CaI2; K2SO4