Determine the minimum concentration of the precipitating agent on the right to cause precipitation of the cation from the solution on the left. b. 0.085 M CaI2; K2SO4

A solution is 0.010 M in Ba2+ and 0.020 M in Ca2+. b. What is the remaining concentration of the cation that precipitates first, when the other cation begins to precipitate?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
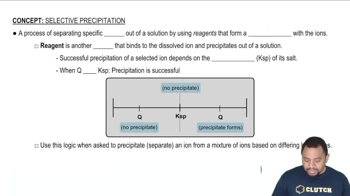
Solubility Product Constant (Ksp)

Precipitation Reaction
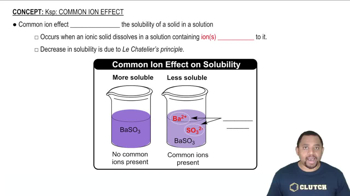
Common Ion Effect
Determine the minimum concentration of the precipitating agent on the right to cause precipitation of the cation from the solution on the left. c. 0.0018 M AgNO3; RbCl
A solution is 0.010 M in Ba2+ and 0.020 M in Ca2+. a. If sodium sulfate is used to selectively precipitate one of the cations while leaving the other cation in solution, which cation will precipitate first? What minimum concentration of Na2SO4 will trigger the precipitation of the cation that precipitates first?
A solution is made 1.1⨉10-3 M in Zn(NO3)2 and 0.150 M in NH3. After the solution reaches equilibrium, what concentration of Zn2+(aq) remains?
Use the appropriate values of Ksp and Kf to find the equilibrium constant for the reaction. FeS(s) + 6 CN-(aq) ⇌ Fe(CN)64-(aq) + S2-(aq)
