Morphine has the formula C17H19NO3. It is a base and accepts one proton per molecule. It is isolated from opium. A 0.682-g sample of opium is found to require 8.92 mL of a 0.0116 M solution of sulfuric acid for neutralization. Assuming that morphine is the only acid or base present in opium, calculate the percent morphine in the sample of opium.
Ch.17 - Acids and Bases

Chapter 17, Problem 143
The pH of a 1.00 M solution of urea, a weak organic base, is 7.050. Calculate the Ka of protonated urea.
 Verified step by step guidance
Verified step by step guidance1
Identify that urea is a weak base and its protonated form acts as a weak acid.
Use the given pH to find the concentration of hydrogen ions \([H^+]\) using the formula \([H^+] = 10^{-\text{pH}}\).
Recognize that the concentration of hydrogen ions \([H^+]\) is equal to the concentration of the conjugate base formed \([A^-]\) in the equilibrium reaction.
Set up the expression for the acid dissociation constant \(K_a\) using the formula \(K_a = \frac{[H^+][A^-]}{[HA]}\), where \([HA]\) is the concentration of the protonated urea.
Assume that the initial concentration of protonated urea is approximately 1.00 M, and solve for \(K_a\) using the concentrations found in the previous steps.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
pH and pKa Relationship
pH is a measure of the hydrogen ion concentration in a solution, defined as pH = -log[H+]. The pKa is the negative logarithm of the acid dissociation constant (Ka) and indicates the strength of an acid. For weak bases like urea, the pH can be used to find the pKa of its conjugate acid, which is essential for calculating Ka.
Recommended video:
Guided course

pH and pOH Calculations
Acid-Base Equilibrium
In acid-base chemistry, equilibrium refers to the state where the rates of the forward and reverse reactions are equal. For protonated urea, the equilibrium can be represented as the dissociation of the conjugate acid into urea and protons. Understanding this equilibrium is crucial for calculating the Ka value, which quantifies the strength of the acid.
Recommended video:
Guided course
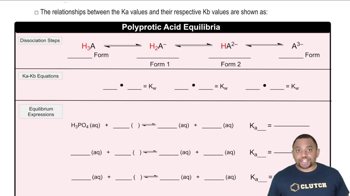
Triprotic Acid Equilibrium
Dissociation Constant (Ka)
The acid dissociation constant (Ka) measures the extent to which an acid can donate protons in solution. A higher Ka value indicates a stronger acid. For protonated urea, calculating Ka involves using the concentrations of the species at equilibrium, which can be derived from the pH of the solution and the initial concentration of urea.
Recommended video:
Guided course
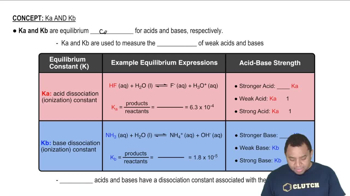
Characteristics of Ka and Kb
Related Practice
Textbook Question
Textbook Question
Determine the pH of each two-component solution. d. 0.088 M HClO4 and 0.022 M KOH
Textbook Question
Write net ionic equations for the reactions that take place when aqueous solutions of the following substances are mixed: a. sodium cyanide and nitric acid b. ammonium chloride and sodium hydroxide c. sodium cyanide and ammonium bromide d. potassium hydrogen sulfate and lithium acetate e. sodium hypochlorite and ammonia
Textbook Question
Lactic acid is a weak acid found in milk. Its calcium salt is a source of calcium for growing animals. A saturated solution of this salt, which we can represent as Ca(Lact)2, has a [Ca2+] = 0.26 M and a pH = 8.78. Assuming the salt is completely dissociated, calculate the Ka of lactic acid.
