Identify each substance as an acid or a base and write a chemical equation showing how it is an acid or a base according to the Arrhenius definition. a. HNO3(aq) b. NH4+(aq) d. HC2H3O2(aq)
Ch.17 - Acids and Bases

Chapter 17, Problem 35a,c
In each reaction, identify the Brønsted–Lowry acid, the Brønsted–Lowry base, the conjugate acid, and the conjugate base. a. H2CO3(aq) + H2O(l) ⇌ H3O+(aq) + HCO3–(aq) c. HNO3(aq) + H2O(l) → H3O+(aq) + NO3–(aq)
 Verified step by step guidance
Verified step by step guidance1
Identify the Brønsted–Lowry acid: Look for the species that donates a proton (H+). In this reaction, H2CO3 donates a proton to H2O.
Identify the Brønsted–Lowry base: Look for the species that accepts a proton. In this reaction, H2O accepts a proton from H2CO3.
Determine the conjugate acid: This is the species formed when the base gains a proton. Here, H3O+ is the conjugate acid formed from H2O.
Determine the conjugate base: This is the species formed when the acid loses a proton. Here, HCO3- is the conjugate base formed from H2CO3.
Summarize the roles: H2CO3 is the Brønsted–Lowry acid, H2O is the Brønsted–Lowry base, H3O+ is the conjugate acid, and HCO3- is the conjugate base.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Brønsted–Lowry Acid-Base Theory
The Brønsted–Lowry theory defines acids as proton donors and bases as proton acceptors. In this framework, an acid releases a hydrogen ion (H+) in a reaction, while a base accepts that ion. This theory expands the understanding of acid-base reactions beyond just the presence of hydroxide ions, allowing for a broader range of chemical interactions.
Recommended video:
Guided course

Bronsted-Lowry Acid-Base Theory
Conjugate Acid and Base
In the context of Brønsted–Lowry theory, a conjugate acid is formed when a base accepts a proton, while a conjugate base is what remains after an acid donates a proton. This relationship is crucial for understanding the reversible nature of acid-base reactions, where the products can also act as acids or bases in subsequent reactions.
Recommended video:
Guided course
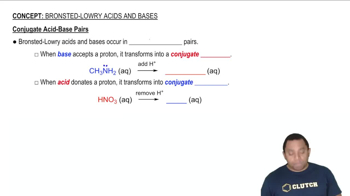
Conjugate Acid-Base Pairs
Identifying Species in Reactions
To analyze acid-base reactions, it is essential to identify the roles of each species involved. In the given reaction, H2CO3 acts as the Brønsted–Lowry acid by donating a proton to H2O, which acts as the Brønsted–Lowry base. The resulting H3O+ is the conjugate acid of water, while HCO3- is the conjugate base of carbonic acid, illustrating the dynamic nature of these chemical species.
Recommended video:
Guided course
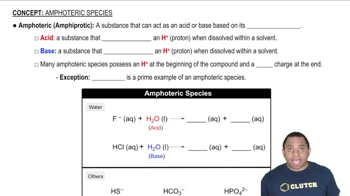
Amphoteric Species
Related Practice
Textbook Question
Textbook Question
Identify each substance as an acid or a base and write a chemical equation showing how it is an acid or a base according to the Arrhenius definition. c. KOH(aq)
1
views
Textbook Question
In each reaction, identify the Brønsted–Lowry acid, the Brønsted–Lowry base, the conjugate acid, and the conjugate base. b. NH3(aq) + H2O(l) ⇌ NH4+(aq) + OH–(aq)
Textbook Question
In each reaction, identify the Brønsted–Lowry acid, the Brønsted–Lowry base, the conjugate acid, and the conjugate base. d. C5H5N(aq) + H2O(l) ⇌ C5H5NH+(aq) + OH–(aq)
Textbook Question
In each reaction, identify the Brønsted–Lowry acid, the Brønsted–Lowry base, the conjugate acid, and the conjugate base. b. CH3NH2(aq) + H2O(l) ⇌ CH3NH3+(aq) + OH–(aq)
