Like all equilibrium constants, the value of Kw depends on temperature. At body temperature (37 °C), Kw = 2.4×10–14. What are the [H3O+] and pH of pure water at body temperature?

Determine whether or not the mixing of each pair of solutions results in a buffer. e. 105.0 mL of 0.15 M CH3NH2; 95.0 mL of 0.10 M HCl
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
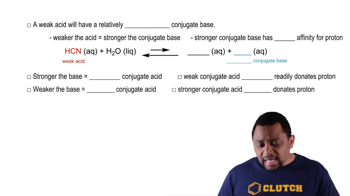
Buffer Solutions

Weak Bases and Their Conjugate Acids
Stoichiometry of Acid-Base Reactions

Complete the table. (All solutions are at 25 °C.)
The value of Kw increases with increasing temperature. Is the autoionization of water endothermic or exothermic?
Calculate the pH of each acid solution. Explain how the resulting pH values demonstrate that the pH of an acid solution should carry as many digits to the right of the decimal place as the number of significant figures in the concentration of the solution. [H3O+] = 0.044 M [H3O+] = 0.045 M [H3O+] = 0.046 M
Determine the concentration of H3O+ to the correct number of significant figures in a solution with each pH. Describe how these calculations show the relationship between the number of digits to the right of the decimal place in pH and the number of significant figures in concentration. pH = 2.50 pH = 2.51 pH = 2.52
For each strong acid solution, determine [H3O+], [OH–], and pH. a. 0.25 M HCl
