Textbook Question
Write equations showing how each weak base ionizes water to form OH–. Also write the corresponding expression for Kb. b. C6H5NH2

 Verified step by step guidance
Verified step by step guidance

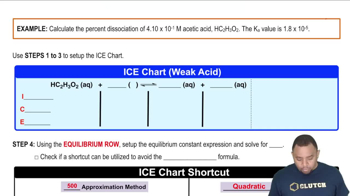
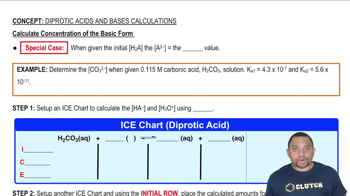
Write equations showing how each weak base ionizes water to form OH–. Also write the corresponding expression for Kb. b. C6H5NH2
For each strong base solution, determine [OH–], [H3O+], pH, and pOH. b. 0.0112 M Ba(OH)2
For each strong base solution, determine [OH–], [H3O+], pH, and pOH. c. 1.9×10–4 M KOH
For each strong base solution, determine [OH–], [H3O+], pH, and pOH. d. 5.0×10–4 M Ca(OH)2
Write equations showing how each weak base ionizes water to form OH–. Also write the corresponding expression for Kb. a. CO32–
Write equations showing how each weak base ionizes water to form OH–. Also write the corresponding expression for Kb. c. C2H5NH2