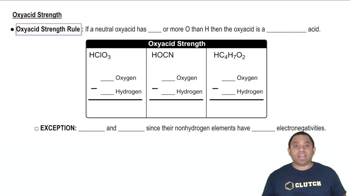
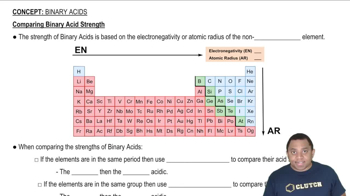
Textbook Question
Consider a 0.10 M solution of a weak polyprotic acid (H2A) with the possible values of Ka1 and Ka2 given here.
a. Ka1 = 1.0 × 10–4; Ka2 = 5.0 × 10–5
Calculate the contributions to [H3O+] from each ionization step. At what point can the contribution of the second step be neglected?