Calculate the concentration of all species in a 0.155 M solution of H2CO3.
Ch.17 - Acids and Bases

Chapter 17, Problem 117
Based on their molecular structure, choose the stronger acid from each pair of binary acids, and explain your choice: a. HF and HCl b. H2O and HF c. H2Se and H2S.
 Verified step by step guidance
Verified step by step guidance1
Identify the periodic trend for acidity in binary acids: Acidity generally increases down a group in the periodic table due to the increase in atomic size, which weakens the H-X bond, making it easier to donate a proton.
For pair (a) HF and HCl: Compare the position of fluorine and chlorine in the periodic table. Chlorine is below fluorine, indicating that HCl is a stronger acid than HF due to the weaker H-Cl bond.
For pair (b) H2O and HF: Consider the electronegativity of the central atom. Fluorine is more electronegative than oxygen, which makes HF a stronger acid than H2O because the H-F bond is more polar, facilitating proton donation.
For pair (c) H2Se and H2S: Compare the position of selenium and sulfur in the periodic table. Selenium is below sulfur, suggesting that H2Se is a stronger acid than H2S due to the larger atomic size of selenium, which weakens the H-Se bond.
Summarize the findings: In each pair, the stronger acid is determined by either the position in the periodic table (for pairs a and c) or the electronegativity of the central atom (for pair b).
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Binary Acids
Binary acids are acids that consist of only two elements, typically hydrogen and a nonmetal. The strength of binary acids is influenced by the bond strength between hydrogen and the nonmetal; weaker bonds lead to stronger acids. For example, in the case of HF and HCl, the H-F bond is stronger than the H-Cl bond, making HF a weaker acid than HCl despite fluorine's high electronegativity.
Recommended video:
Guided course
Binary Acids
Electronegativity
Electronegativity is the tendency of an atom to attract electrons in a chemical bond. In binary acids, the electronegativity of the nonmetal affects the acid's strength; higher electronegativity typically leads to stronger acids due to the greater polarity of the H-X bond. For instance, in comparing HF and HCl, while HF has a highly electronegative fluorine, the bond strength ultimately results in HCl being the stronger acid.
Recommended video:
Guided course

Electronegativity Trends
Bond Strength and Acid Strength
The strength of the bond between hydrogen and the nonmetal in a binary acid directly impacts the acid's strength. Weaker bonds are more easily broken, allowing the acid to donate protons (H+) more readily. For example, in the pair H2Se and H2S, the H-Se bond is weaker than the H-S bond, making H2Se the stronger acid because it can more easily release a proton.
Recommended video:
Guided course
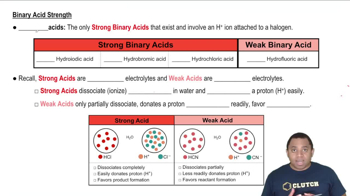
Binary Acid Strengths
Related Practice
Textbook Question
Textbook Question
Calculate the [H3O+] and pH of each H2SO4 solution. At approximately what concentration does the x is small approximation break down?
a. 0.50 M b. 0.10 M c. 0.050 M
Textbook Question
Consider a 0.10 M solution of a weak polyprotic acid (H2A) with the possible values of Ka1 and Ka2 given here.
a. Ka1 = 1.0 × 10–4; Ka2 = 5.0 × 10–5
Calculate the contributions to [H3O+] from each ionization step. At what point can the contribution of the second step be neglected?
Textbook Question
Based on molecular structure, arrange the binary compounds in order of increasing acid strength. Explain your choice. H2Te, HI, H2S, NaH
Textbook Question
Based on molecular structure, arrange the oxyacids in order of increasing acid strength. Explain your choice. HClO3, HIO3, HBrO3
