Textbook Question
The tabulated data were collected for this reaction at 500 °C: CH3CN(g) → CH3NC( g) b. What is the half-life for this reaction (at the initial concentration)?

 Verified step by step guidance
Verified step by step guidance

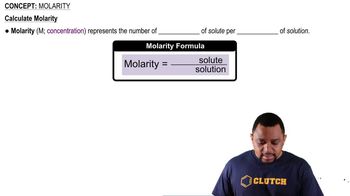
The tabulated data were collected for this reaction at 500 °C: CH3CN(g) → CH3NC( g) b. What is the half-life for this reaction (at the initial concentration)?
The tabulated data were collected for this reaction at a certain temperature: X2Y → 2 X + Y a. Determine the order of the reaction and the value of the rate constant at this temperature.
Consider the reaction: 2 O3(g) → 3 O2( g) The rate law for this reaction is: Rate = k [O3]2 [O2] Suppose that a 1.0-L reaction vessel initially contains 1.0 mol of O3 and 1.0 mol of O2. What fraction of the O3 will have reacted when the rate falls to one-half of its initial value?