Consider the gas-phase reaction: H2(g) + I2(g) → 2 HI(g) The reaction was experimentally determined to be first order in H2 and first order in I2. Consider the proposed mechanisms. Proposed mechanism I: H2(g) + I2(g) → 2 HI(g) Single step Proposed mechanism II: I2(g) Δk1k-12 I(g) Fast H2( g) + 2 I( g) → k22 HI( g) Slow a. Show that both of the proposed mechanisms are valid.
Ch.15 - Chemical Kinetics

Chapter 15, Problem 106
Phosgene (Cl2CO), a poison gas used in World War I, is formed
by the reaction of Cl2 and CO. The proposed mechanism for the
reaction is:
Cl2Δ2 Cl (fast, equilibrium)
Cl + COΔClCO (fast, equilibrium)
ClCO + Cl2¡Cl2CO + Cl (slow)
 Verified step by step guidance
Verified step by step guidance1
Identify the overall reaction by summing up the elementary steps in the proposed mechanism.
Determine the rate-determining step, which is the slowest step in the mechanism.
Write the rate law based on the rate-determining step, using the concentrations of the reactants involved in that step.
Since the rate-determining step involves intermediates, express the concentration of the intermediate in terms of the reactants using the fast equilibrium steps.
Substitute the expression for the intermediate back into the rate law to obtain the rate law in terms of the initial reactants.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Reaction Mechanism
A reaction mechanism is a step-by-step description of the process by which reactants are converted into products. It outlines the individual elementary steps involved, including the formation and consumption of intermediates. Understanding the mechanism helps in predicting the rate of reaction and the effect of various conditions on the reaction's progress.
Recommended video:
Guided course
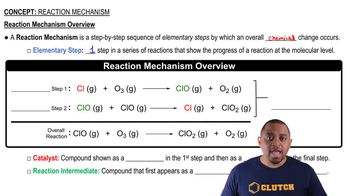
Reaction Mechanism Overview
Equilibrium
Equilibrium in a chemical reaction refers to the state where the rates of the forward and reverse reactions are equal, resulting in constant concentrations of reactants and products. In the context of the proposed mechanism, the fast steps are at equilibrium, meaning they can be represented by equilibrium constants, which influence the overall reaction dynamics.
Recommended video:
Guided course
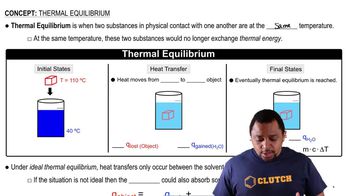
Thermal Equilibrium
Rate-Determining Step
The rate-determining step (RDS) is the slowest step in a reaction mechanism that controls the overall reaction rate. In the provided mechanism, the last step is identified as the slow step, indicating that it has the highest activation energy and thus dictates how quickly the reaction proceeds. Understanding the RDS is crucial for optimizing reaction conditions and improving yields.
Recommended video:
Guided course

Rate Law Determination
Related Practice
Textbook Question
Textbook Question
Consider the gas-phase reaction: H2(g) + I2(g) → 2 HI(g) The reaction was experimentally determined to be first order in H2 and first order in I2. Consider the proposed mechanisms. Proposed mechanism I: H2(g) + I2(g) → 2 HI(g) Single step Proposed mechanism II: I2(g) Δk1k-12 I(g) Fast H2( g) + 2 I( g) → k22 HI( g) Slow b. What kind of experimental evidence might lead you to favor mechanism II over mechanism I?
Textbook Question
A certain substance X decomposes. Fifty percent of X remains after 100 minutes. How much X remains after 200 minutes if the reaction order with respect to X is (c) second order?
