A reaction has a rate constant of 0.000122/s at 27 °C and 0.228/s at 77 °C. b. What is the value of the rate constant at 17 °C?
Ch.15 - Chemical Kinetics

Chapter 15, Problem 69
What is the value of the rate constant at 425 K for a reaction with rate constants of 0.0117/s at 400.0 K and 0.689/s at 450.0 K?
 Verified step by step guidance
Verified step by step guidance1
Identify that the problem involves calculating the rate constant at a specific temperature using the Arrhenius equation.
Recall the Arrhenius equation: \( k = A e^{-\frac{E_a}{RT}} \), where \( k \) is the rate constant, \( A \) is the pre-exponential factor, \( E_a \) is the activation energy, \( R \) is the gas constant, and \( T \) is the temperature in Kelvin.
Use the two given rate constants and temperatures to set up two equations based on the Arrhenius equation: \( \ln(k_1) = \ln(A) - \frac{E_a}{R} \cdot \frac{1}{T_1} \) and \( \ln(k_2) = \ln(A) - \frac{E_a}{R} \cdot \frac{1}{T_2} \).
Subtract the first equation from the second to eliminate \( \ln(A) \) and solve for \( E_a \): \( \ln(k_2) - \ln(k_1) = -\frac{E_a}{R} \left( \frac{1}{T_2} - \frac{1}{T_1} \right) \).
Once \( E_a \) is found, use it in the Arrhenius equation to solve for the rate constant \( k \) at 425 K: \( \ln(k) = \ln(A) - \frac{E_a}{R} \cdot \frac{1}{425} \).
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Arrhenius Equation
The Arrhenius equation describes how the rate constant of a reaction depends on temperature. It is expressed as k = A * e^(-Ea/RT), where k is the rate constant, A is the pre-exponential factor, Ea is the activation energy, R is the universal gas constant, and T is the temperature in Kelvin. This relationship indicates that as temperature increases, the rate constant typically increases due to higher molecular energy and more frequent effective collisions.
Recommended video:
Guided course
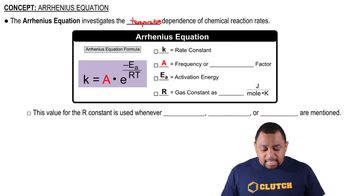
Arrhenius Equation
Temperature Dependence of Rate Constants
Rate constants are temperature-dependent, meaning they change with variations in temperature. This dependence can be analyzed using the Arrhenius equation or by comparing rate constants at different temperatures. In this question, the rate constants at 400 K and 450 K are used to estimate the rate constant at an intermediate temperature of 425 K, highlighting the importance of understanding how temperature influences reaction kinetics.
Recommended video:
Guided course
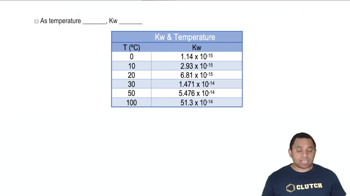
Kw Temperature Dependence
Linear Interpolation
Linear interpolation is a mathematical method used to estimate unknown values that fall within the range of known data points. In this context, it can be applied to find the rate constant at 425 K by using the known rate constants at 400 K and 450 K. This technique assumes a linear relationship between the two known points, allowing for a straightforward calculation of the desired value.
Recommended video:
Guided course
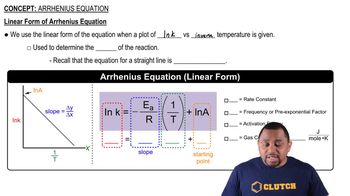
Linear Form of Arrhenius Equation
Related Practice
Textbook Question
Textbook Question
The data shown here were collected for the first-order reaction: N2O(g) → N2(g) + O(g) Use an Arrhenius plot to determine the activation barrier and frequency factor for the reaction.
Temperature (K) Rate Constant (1 , s)
800 3.24⨉10- 5
900 0.00214
1000 0.0614
1100 0.955
Textbook Question
The tabulated data show the rate constant of a reaction measured at several different temperatures. Use an Arrhenius plot to determine the activation barrier and frequency factor for the reaction.
Temperature (K) Rate Constant (1 , s)
300 0.0134
310 0.0407
320 0.114
330 0.303
340 0.757
Textbook Question
A reaction has a rate constant of 0.0117/s at 400.0 K and 0.689/s at 450.0 K. a. Determine the activation barrier for the reaction.
1
views
