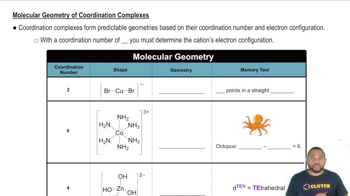
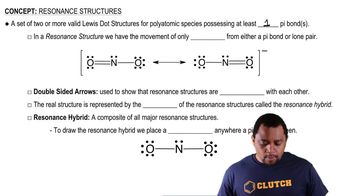
Textbook Question
An unknown metal is found to have a density of 7.8748 g/cm3 and to crystallize in a body-centered cubic lattice. The edge of the unit cell is 0.28664 nm. Calculate the atomic mass of the metal.
1
views

 Verified step by step guidance
Verified step by step guidance
An unknown metal is found to have a density of 7.8748 g/cm3 and to crystallize in a body-centered cubic lattice. The edge of the unit cell is 0.28664 nm. Calculate the atomic mass of the metal.
Calculate the fraction of empty space in cubic closest packing to five significant figures.
X-ray diffractometers often use metals that have had their core electrons excited as a source of X-rays. Consider the 2p → 1s transition for copper, which is called the K⍺ transition. Calculate the wavelength of X-rays (in Å) given off by the K⍺ transition if the energy given off by a mole of copper atoms is 7.77⨉105 kJ.(1Å = 10-10 m)