Ethanol has a heat of vaporization of 38.56 kJ/mol and a normal boiling point of 78.4 °C. What is the vapor pressure of ethanol at 15 °C?
Ch.12 - Liquids, Solids & Intermolecular Forces

Chapter 12, Problem 66
Methylamine has a vapor pressure of 344 torr at -25 °C and a boiling point of -6.4 °C. What is the ΔHvap for methylamine?
 Verified step by step guidance
Verified step by step guidance1
Identify the Clausius-Clapeyron equation: \( \ln \left( \frac{P_1}{P_2} \right) = \frac{\Delta H_{\text{vap}}}{R} \left( \frac{1}{T_2} - \frac{1}{T_1} \right) \), where \( P_1 \) and \( P_2 \) are the vapor pressures at temperatures \( T_1 \) and \( T_2 \), respectively, and \( R \) is the ideal gas constant.
Convert the temperatures from Celsius to Kelvin by adding 273.15 to each temperature. Thus, \( T_1 = -25 + 273.15 \) K and \( T_2 = -6.4 + 273.15 \) K.
Recognize that at the boiling point, the vapor pressure \( P_2 \) is equal to 1 atm (or 760 torr). Therefore, \( P_1 = 344 \) torr and \( P_2 = 760 \) torr.
Substitute the known values into the Clausius-Clapeyron equation: \( \ln \left( \frac{344}{760} \right) = \frac{\Delta H_{\text{vap}}}{R} \left( \frac{1}{T_2} - \frac{1}{T_1} \right) \).
Solve for \( \Delta H_{\text{vap}} \) by rearranging the equation: \( \Delta H_{\text{vap}} = R \cdot \ln \left( \frac{344}{760} \right) \cdot \left( \frac{1}{T_2} - \frac{1}{T_1} \right)^{-1} \).
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Vapor Pressure
Vapor pressure is the pressure exerted by a vapor in equilibrium with its liquid or solid phase at a given temperature. It reflects the tendency of particles to escape from the liquid phase into the gas phase. Higher vapor pressure indicates a greater number of molecules escaping into the vapor phase, which is crucial for understanding the boiling point and the enthalpy of vaporization.
Recommended video:
Guided course

Raoult's Law and Vapor Pressure
Boiling Point
The boiling point of a substance is the temperature at which its vapor pressure equals the external pressure surrounding the liquid. At this point, the liquid transitions to a gas. For methylamine, the boiling point of -6.4 °C indicates the temperature at which it can vaporize under standard atmospheric pressure, providing insight into its physical properties and behavior.
Recommended video:
Guided course

Boiling Point Elevation
Enthalpy of Vaporization (ΔHvap)
The enthalpy of vaporization (ΔHvap) is the amount of energy required to convert a unit mass of a liquid into vapor at constant temperature and pressure. It is a critical thermodynamic property that can be calculated using the Clausius-Clapeyron equation, which relates vapor pressure and temperature, allowing for the determination of ΔHvap from known vapor pressures at different temperatures.
Recommended video:
Guided course
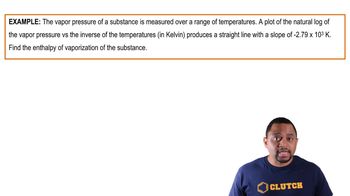
Enthalpy of Vaporization Example
Related Practice
Textbook Question
2
views
Textbook Question
Benzene has a heat of vaporization of 30.72 kJ/mol and a normal boiling point of 80.1 °C. At what temperature does benzene boil when the external pressure is 445 torr?
Textbook Question
Carbon disulfide has a vapor pressure of 363 torr at 25 °C and a normal boiling point of 46.3 °C. Find ΔHvap for carbon disulfide.
1
views
Textbook Question
How much energy is released when 65.8 g of water freezes?
Textbook Question
Calculate the amount of heat required to completely sublime 50.0 g of solid dry ice (CO2) at its sublimation temperature. The heat of sublimation for carbon dioxide is 32.3 kJ/mol.
Textbook Question
An 8.5-g ice cube is placed into 255 g of water. Calculate the temperature change in the water upon the complete melting of the ice. Assume that all of the energy required to melt the ice comes from the water.
