Draw an MO energy diagram and predict the bond order of Li2+ and Li2-. Do you expect these molecules to exist in the gas phase?

 Tro 5th Edition
Tro 5th Edition Ch.11 - Chemical Bonding II: Molecular Shapes, VSEPR & MO Theory
Ch.11 - Chemical Bonding II: Molecular Shapes, VSEPR & MO Theory Problem 75a
Problem 75aUsing the molecular orbital energy ordering for second-row homonuclear diatomic molecules in which the π2p orbitals lie at lower energy than the σ2p, draw MO energy diagrams and predict the bond order in a molecule or ion with each number of total valence electrons. Will the molecule or ion be diamagnetic or paramagnetic? a. 4 b. 6
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
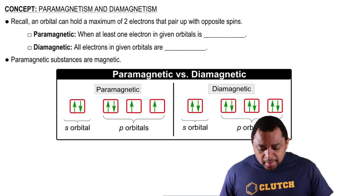
Key Concepts
Molecular Orbital Theory

Bond Order

Diamagnetism and Paramagnetism
Sketch the bonding and antibonding molecular orbitals that result from linear combinations of the 2px atomic orbitals in a homonuclear diatomic molecule. (The 2px orbitals are those whose lobes are oriented along the bonding axis.)
Sketch the bonding and antibonding molecular orbitals that result from linear combinations of the 2pz atomic orbitals in a homonuclear diatomic molecule. (The 2pz orbitals are those whose lobes are oriented perpendicular to the bonding axis.) How do these molecular orbitals differ from those obtained from linear combinations of the 2py atomic orbitals? (The 2py orbitals are also oriented perpendicular to the bonding axis, but also perpendicular to the 2pz orbitals.)
Using the molecular orbital energy ordering for second-row homonuclear diatomic molecules in which the π2p orbitals lie at lower energy than the σ2p, draw MO energy diagrams and predict the bond order in a molecule or ion with each number of total valence electrons. Will the molecule or ion be diamagnetic or paramagnetic? c. 8
Using the molecular orbital energy ordering for second-row homonuclear diatomic molecules in which the π2p orbitals lie at lower energy than the σ2p, draw MO energy diagrams and predict the bond order in a molecule or ion with each number of total valence electrons. Will the molecule or ion be diamagnetic or paramagnetic?? b. 6 d. 9