A sample of gaseous neon atoms at atmospheric pressure and 0 °C contains 2.69×1022 atoms per liter. The atomic radius of neon is 69 pm. What fraction of the space do the atoms themselves occupy? What does this reveal about the separation between atoms in the gaseous phase?

Lead metal can be extracted from a mineral called galena, which contains 86.6% lead by mass. A particular ore contains 68.5% galena by mass. If the lead can be extracted with 92.5% efficiency, what mass of ore is required to make a lead sphere with a 5.00-cm radius?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Mass Percent Composition

Volume of a Sphere
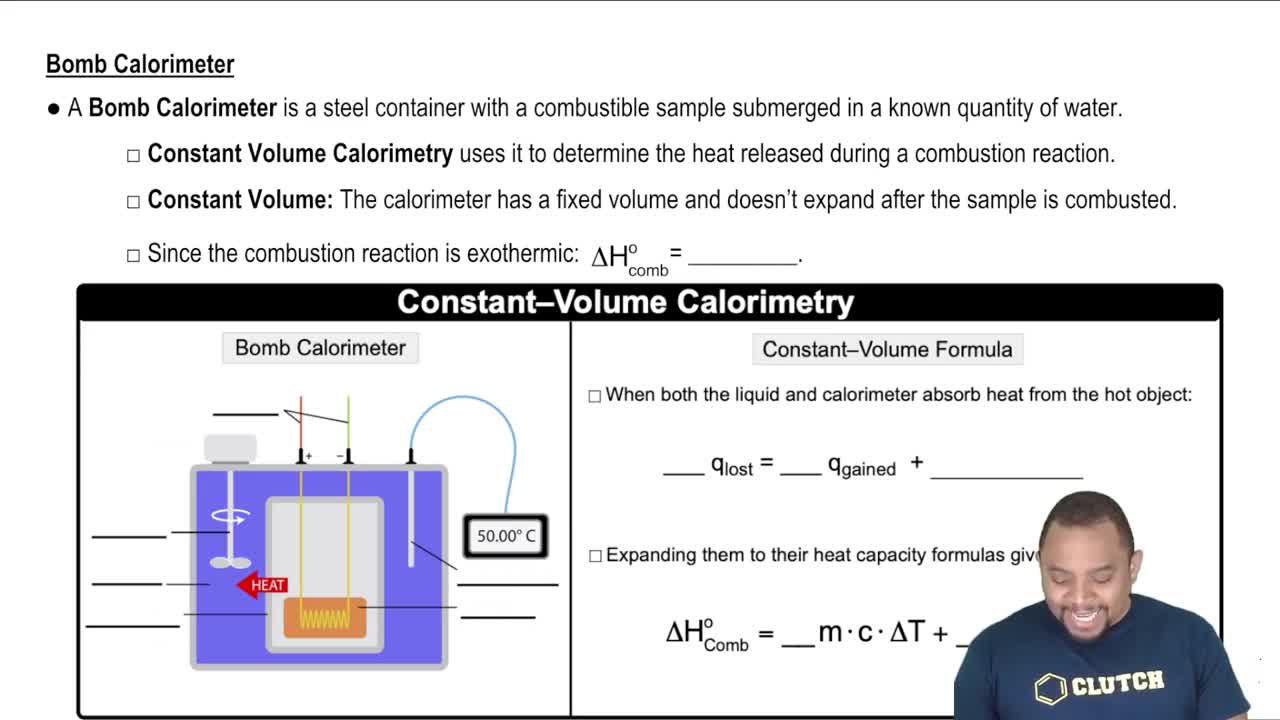
Extraction Efficiency
The diameter of a hydrogen atom is 212 pm. Find the length in kilometers of a row of 6.02×1023 hydrogen atoms. The diameter of a ping pong ball is 4.0 cm. Find the length in kilometers of a row of 6.02×1023 ping pong balls.
The daily recommended intake of calcium for an average adult is 1,000 mg. There is 125 mg of calcium in 100 grams of milk. If a 150 g smoothie contains 75 grams of milk, how many grams of smoothie should an adult consume to meet the daily recommended intake?
A length of #8 copper wire (radius = 1.63 mm) has a mass of 24.0 kg and a resistance of 2.061 ohm per km (Ω / km). What is the overall resistance of the wire?
Rolls of aluminum foil are 304 mm wide and 0.016 mm thick. What maximum length of aluminum foil can be made from 1.10 kg of aluminum?
