Textbook Question
Hydrogenation reactions are used to add hydrogen across double bonds in hydrocarbons and other organic compounds. Use average bond energies to calculate ΔHrxn for the hydrogenation reaction. H2C=CH2(g) + H2(g) → H3C–CH3(g)
1
views

 Verified step by step guidance
Verified step by step guidance
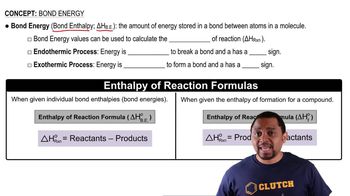

Hydrogenation reactions are used to add hydrogen across double bonds in hydrocarbons and other organic compounds. Use average bond energies to calculate ΔHrxn for the hydrogenation reaction. H2C=CH2(g) + H2(g) → H3C–CH3(g)
Ethanol is a possible fuel. Use average bond energies to calculate ΔHrxn for the combustion of ethanol. CH3CH2OH(g) + 3 O2(g) → 2 CO2(g) + 3 H2O(g)
Write an appropriate Lewis structure for each compound. Make certain to distinguish between ionic and molecular compounds. b. ClF5
Each compound contains both ionic and covalent bonds. Write ionic Lewis structures for each, including the covalent structure for the ion in brackets. Write resonance structures if necessary. a. BaCO3