Textbook Question
Determine if a bond between each pair of atoms would be pure covalent, polar covalent, or ionic. a. Br and Br

 Verified step by step guidance
Verified step by step guidance

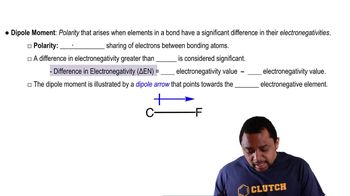
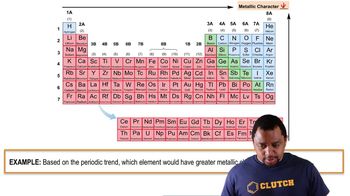
Determine if a bond between each pair of atoms would be pure covalent, polar covalent, or ionic. a. Br and Br
Refer to Figure 9.10 to estimate the percent ionic character of the CO bond.
Refer to Figure 9.10 to estimate the percent ionic character of the BrF bond.
Write the Lewis structure for each molecule or ion. a. CI4 b. N2O c. SiH4 d. Cl2CO
Write the Lewis structure for each molecule or ion. b. OH-