Textbook Question
Which of the transition elements in the first transition series have anomalous electron configurations?

 Verified step by step guidance
Verified step by step guidance

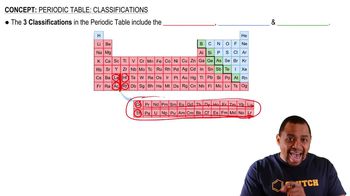

Which of the transition elements in the first transition series have anomalous electron configurations?
Write the full electron configuration for each element. a. Si b. O c. K d. Ne
Write the full orbital diagram for each element. a. N b. F c. Mg d. Al
Write the full orbital diagram for each element. a. S
Write the full orbital diagram for each element. b. Ca