Textbook Question
The elements with atomic numbers 35 and 53 have similar chemical properties. Based on their electronic configurations, predict the atomic number of a heavier element that also should share these chemical properties.

 Verified step by step guidance
Verified step by step guidance
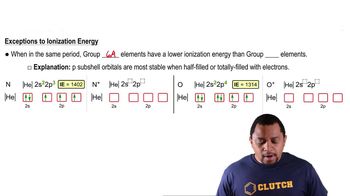
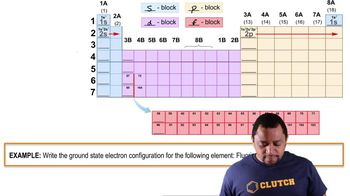
The elements with atomic numbers 35 and 53 have similar chemical properties. Based on their electronic configurations, predict the atomic number of a heavier element that also should share these chemical properties.
You have cracked a secret code that uses elemental symbols to spell words. The code uses numbers to designate the elemental symbols. Each number is the sum of the atomic number and the highest principal quantum number of the highest occupied orbital of the element whose symbol is to be used. The message may be written forward or backward. Decode the following messages: a. 10, 12, 58, 11, 7, 44, 63, 66