The speed of sound in air is 344 m/s at room temperature. The lowest frequency of a large organ pipe is 30 s–1 and the highest frequency of a piccolo is 1.5×104 s–1. Find the difference in wavelength between these two sounds.
Ch.7 - Quantum-Mechanical Model of the Atom

Chapter 7, Problem 89
The iodine molecule can be photodissociated (broken apart with light) into iodine atoms in the gas phase with light of wavelengths shorter than about 792 nm. A 100.0-mL glass tube contains 55.7 mtorr of gaseous iodine at 25.0 °C. What is the minimum amount of light energy that must be absorbed by the iodine in the tube to dissociate 15.0% of the molecules?
 Verified step by step guidance
Verified step by step guidance1
Convert the pressure from mtorr to atm by using the conversion factor: 1 atm = 760 torr.
Use the ideal gas law, PV = nRT, to calculate the number of moles of iodine gas (I2) in the tube. Remember to convert the volume to liters and use the appropriate value for R (0.0821 L·atm/mol·K).
Calculate the number of moles of iodine molecules that need to be dissociated by multiplying the total moles by 15.0%.
Determine the energy required to dissociate one mole of iodine molecules using the wavelength of light (792 nm) and the equation E = hc/λ, where h is Planck's constant and c is the speed of light.
Calculate the total energy required to dissociate the calculated moles of iodine molecules by multiplying the energy per mole by the number of moles to be dissociated.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Photodissociation
Photodissociation is a process in which a chemical bond is broken due to the absorption of light energy. In the case of iodine, when exposed to light with wavelengths shorter than 792 nm, the energy from the photons can break the I-I bond, resulting in the formation of individual iodine atoms. Understanding this concept is crucial for calculating the energy required for dissociation.
Energy of Photons
The energy of a photon is inversely related to its wavelength, described by the equation E = hc/λ, where E is energy, h is Planck's constant, c is the speed of light, and λ is the wavelength. This relationship is essential for determining the minimum energy needed to dissociate iodine molecules, as it allows us to calculate the energy associated with the light that can break the bonds in the iodine molecule.
Recommended video:
Guided course
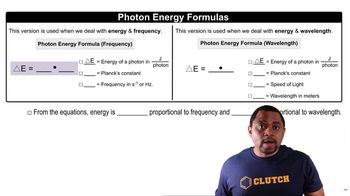
Photon Energy Formulas
Partial Pressure and Mole Fraction
Partial pressure refers to the pressure exerted by a single component of a gas mixture, while mole fraction is the ratio of the number of moles of a component to the total number of moles in the mixture. In this scenario, knowing the partial pressure of iodine helps in calculating the number of molecules present, which is necessary to determine how many need to be dissociated to achieve the desired percentage.
Recommended video:
Guided course

Mole Fraction and Partial Pressure Calculation Example
Related Practice
Textbook Question
2
views
Textbook Question
The distance from Earth to the sun is 1.5×108 km. Find the number of crests in a light wave of frequency 1.0×1014 s –1 traveling from the sun to Earth.
Textbook Question
A 5.00-mL ampule of a 0.100-M solution of naphthalene in hexane is excited with a flash of light. The naphthalene emits 15.5 J of energy at an average wavelength of 349 nm. What percentage of the naphthalene molecules emitted a photon?
1
views
Textbook Question
A laser produces 20.0 mW of red light. In 1.00 hr, the laser emits 2.29×1020 photons. What is the wavelength of the laser?
1
views
Textbook Question
A particular laser consumes 150.0 watts of electrical power and produces a stream of 1.33×1019 1064-nm photons per second. What is the percent efficiency of the laser in converting electrical power to light?
1
views
