Under certain nonstandard conditions, oxidation by O2(g) of 1 mol of SO2(g) to SO3(g) absorbs 89.5 kJ. The enthalpy of formation of SO3(g) is –204.2 kJ under these conditions. Find the enthalpy of formation of SO2(g).
Ch.6 - Thermochemistry

Chapter 6, Problem 110
A mixture of 2.0 mol of H2(g) and 1.0 mol of O2(g) is placed in a sealed evacuated container made of a perfect insulating material at 25 °C. The mixture is ignited with a spark and reacts to form liquid water. Determine the temperature of the water.
 Verified step by step guidance
Verified step by step guidance1
Write the balanced chemical equation for the reaction between hydrogen gas (H2) and oxygen gas (O2) to form liquid water (H2O). The balanced equation is: 2 H2(g) + O2(g) → 2 H2O(l).
Calculate the limiting reactant. Since the reaction consumes hydrogen and oxygen in a 2:1 ratio, compare the moles of H2 and O2 provided to determine which is the limiting reactant.
Use stoichiometry to determine the amount of water produced based on the limiting reactant. This involves using the mole ratio from the balanced equation.
Calculate the heat released during the reaction. Use the enthalpy change of the reaction (ΔH) for the formation of water from hydrogen and oxygen gases. The ΔH for the formation of liquid water from H2(g) and O2(g) is typically found in tables of standard enthalpies of formation.
Apply the concept of conservation of energy (the first law of thermodynamics) to determine the final temperature of the water. Since the container is insulated, all the heat released from the reaction will be used to increase the temperature of the water formed. Use the specific heat capacity of water and the mass of water formed to calculate the final temperature.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Stoichiometry of Combustion Reactions
Stoichiometry involves the calculation of reactants and products in chemical reactions. In this case, the combustion of hydrogen (H2) with oxygen (O2) follows the balanced equation 2H2 + O2 → 2H2O. Understanding the mole ratios is essential to determine how much water is produced and the energy released during the reaction.
Recommended video:
Guided course
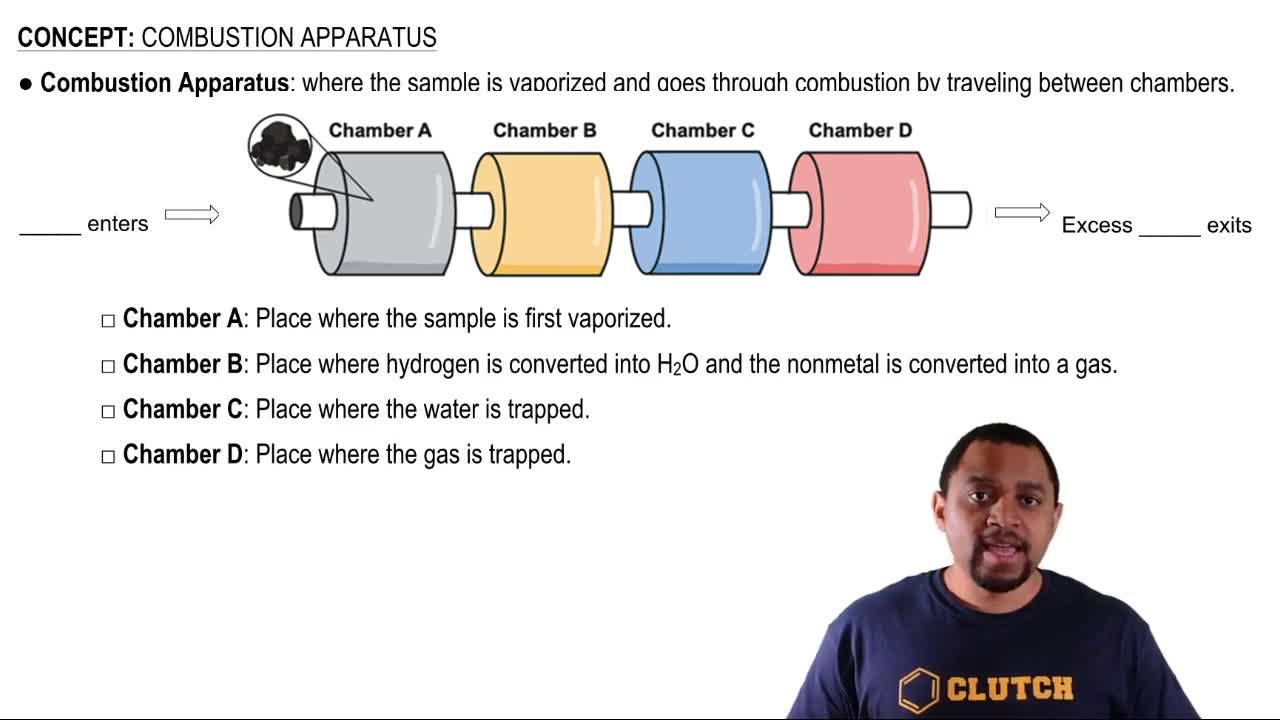
Combustion Apparatus
Thermodynamics and Heat Transfer
Thermodynamics studies the relationships between heat, work, and energy. In this scenario, the reaction is exothermic, meaning it releases heat. The temperature of the water formed will depend on the amount of heat released and the specific heat capacity of water, which dictates how much the temperature will rise based on the energy transferred.
Recommended video:
Guided course

First Law of Thermodynamics
Ideal Gas Law and Reaction Conditions
The Ideal Gas Law (PV=nRT) relates the pressure, volume, temperature, and number of moles of a gas. Although the gases are ignited in a sealed container, understanding the initial conditions (25 °C) and the behavior of gases during the reaction is crucial for predicting the final state of the products, including the temperature of the water formed.
Recommended video:
Guided course

Ideal Gas Law Formula
Related Practice
Textbook Question
1
views
Textbook Question
A 20.0-L volume of an ideal gas in a cylinder with a piston is at a pressure of 3.0 atm. Enough weight is suddenly removed from the piston to lower the external pressure to 1.5 atm. The gas then expands at constant temperature until its pressure is 1.5 atm. Find w.
17
views
Textbook Question
When 10.00 g of phosphorus is burned in O2(g) to form P4O10(s), enough heat is generated to raise the temperature of 2950 g of water from 18.0 °C to 38.0 °C. Calculate the enthalpy of formation of P4O10(s) under these conditions.
Textbook Question
The ΔH for the oxidation of sulfur in the gas phase to SO3 is –204 kJ/mol and for the oxidation of SO2 to SO3 is 89.5 kJ/mol. Find the enthalpy of formation of SO2 under these conditions.
