The pressure in Denver, Colorado (elevation 5280 ft), averages about 24.9 in Hg. Convert this pressure to each indicated unit. b. mmHg
Ch.5 - Gases

Chapter 5, Problem 26
The pressure on top of Mount Everest (29,029 ft) averages about 235 mmHg. Convert this pressure to each indicated unit. a. torr b. psi c. in Hg d. atm
 Verified step by step guidance
Verified step by step guidance1
insert step 1: Understand that 1 mmHg is equivalent to 1 torr, so the pressure in torr is the same as in mmHg.
insert step 2: To convert mmHg to psi, use the conversion factor: 1 mmHg = 0.0193368 psi. Multiply 235 mmHg by this factor.
insert step 3: To convert mmHg to inches of mercury (in Hg), use the conversion factor: 1 mmHg = 0.0393701 in Hg. Multiply 235 mmHg by this factor.
insert step 4: To convert mmHg to atmospheres (atm), use the conversion factor: 1 atm = 760 mmHg. Divide 235 mmHg by 760.
insert step 5: Review each conversion to ensure the correct application of conversion factors and units.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
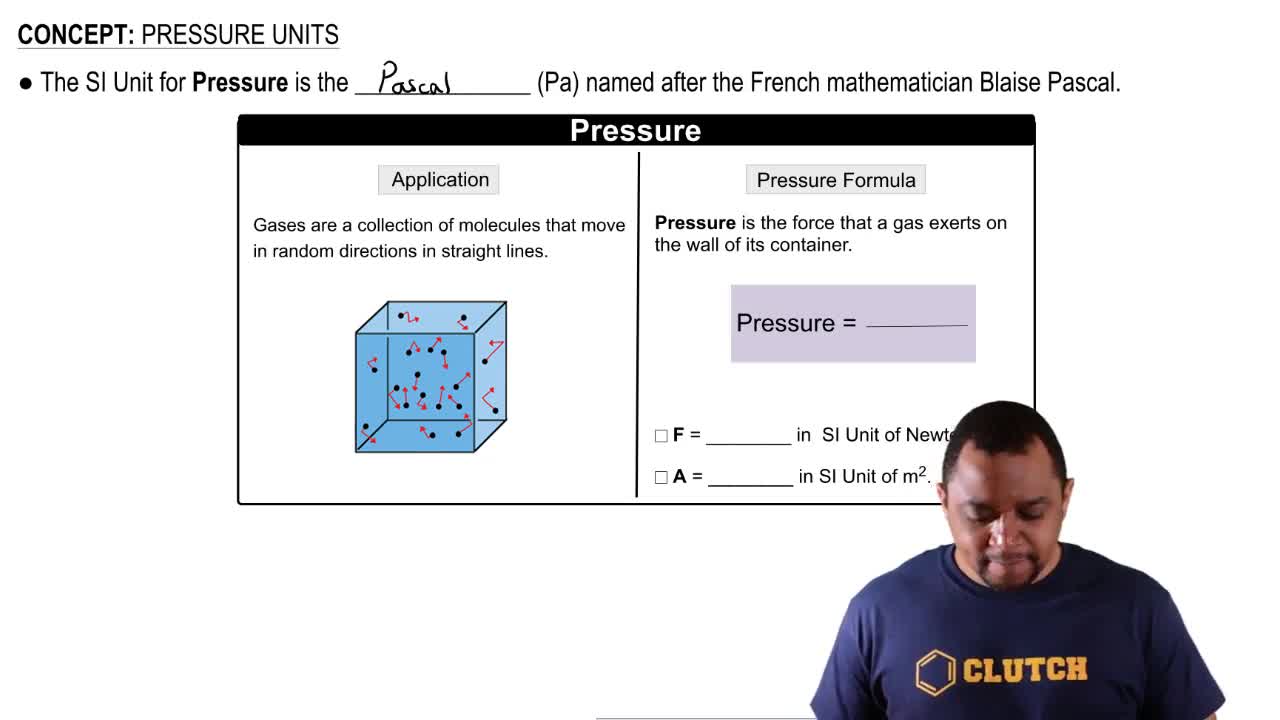
Pressure Units
Pressure is a measure of force applied per unit area and can be expressed in various units, including mmHg, torr, psi, inHg, and atm. Understanding the relationships between these units is essential for converting pressure values. For instance, 1 torr is equivalent to 1 mmHg, while 1 atm is defined as 760 mmHg.
Recommended video:
Guided course
Pressure Units
Unit Conversion
Unit conversion involves changing a measurement from one unit to another while maintaining the same quantity. This process often requires conversion factors, which are ratios that express how many of one unit are equivalent to another. For example, to convert mmHg to atm, one would use the factor that 1 atm equals 760 mmHg.
Recommended video:
Guided course

Conversion Factors
Atmospheric Pressure
Atmospheric pressure is the pressure exerted by the weight of the atmosphere above a given point. At sea level, standard atmospheric pressure is defined as 1 atm, which is approximately 101.3 kPa or 760 mmHg. Understanding atmospheric pressure is crucial for interpreting pressure readings at different altitudes, such as on Mount Everest.
Recommended video:
Guided course

Total Pressure Example
Related Practice
Textbook Question
1
views
1
rank
Textbook Question
The pressure in Denver, Colorado (elevation 5280 ft), averages about 24.9 in Hg. Convert this pressure to each indicated unit. c. psi
2
views
1
rank
Textbook Question
The pressure in Denver, Colorado (elevation 5280 ft), averages about 24.9 in Hg. Convert this pressure to each indicated unit. d. Pa
1
views
Textbook Question
The North American record for highest recorded barometric pressure is 31.85 in Hg, set in 1989 in Northway, Alaska. Convert this pressure to each indicated unit. a. mmHg
1
views
Textbook Question
The world record for lowest pressure (at sea level) was 652.5 mmHg recorded inside Typhoon Tip on October 12, 1979, in the western Pacific Ocean. Convert this pressure to each indicated unit. a. torr c. in Hg
