A mixture of 8.0 g CH4 and 8.0 g Xe is placed in a container and the total pressure is found to be 0.44 atm. Determine the partial pressure of CH4.
Ch.5 - Gases

Chapter 5, Problem 125
In a given diffusion apparatus, 15.0 mL of HBr gas diffuses in 1.0 min. In the same apparatus and under the same conditions, 20.3 mL of an unknown gas diffuses in 1.0 min. The unknown gas is a hydrocarbon. Find its molecular formula.
 Verified step by step guidance
Verified step by step guidance1
Use Graham's law of effusion, which states that the rate of effusion of a gas is inversely proportional to the square root of its molar mass: \( \frac{r_1}{r_2} = \sqrt{\frac{M_2}{M_1}} \).
Identify the known values: \( r_1 = 15.0 \text{ mL/min} \) for HBr and \( r_2 = 20.3 \text{ mL/min} \) for the unknown gas. The molar mass of HBr (\( M_1 \)) is approximately 80.91 g/mol.
Rearrange Graham's law to solve for the molar mass of the unknown gas (\( M_2 \)): \( M_2 = M_1 \left( \frac{r_1}{r_2} \right)^2 \).
Substitute the known values into the equation to calculate \( M_2 \).
Determine the molecular formula of the hydrocarbon by comparing the calculated molar mass to the molar masses of possible hydrocarbons (e.g., CH₄, C₂H₆, C₃H₈, etc.).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
5mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Graham's Law of Effusion
Graham's Law states that the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass. This means that lighter gases diffuse faster than heavier gases. In this problem, the relationship between the diffusion rates of HBr and the unknown hydrocarbon can be used to determine the molar mass of the unknown gas.
Recommended video:
Guided course
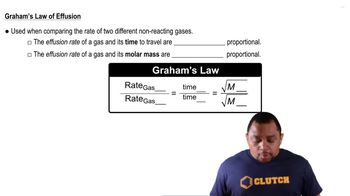
Graham's Law of Effusion
Molar Mass Calculation
Molar mass is the mass of one mole of a substance, typically expressed in grams per mole (g/mol). To find the molecular formula of the unknown hydrocarbon, we need to calculate its molar mass based on the diffusion rates provided. By applying Graham's Law, we can derive the molar mass of the unknown gas from the known molar mass of HBr.
Recommended video:
Guided course

Molar Mass Calculation Example
Hydrocarbon Structure
Hydrocarbons are organic compounds consisting entirely of hydrogen and carbon. They can be classified into alkanes, alkenes, and alkynes based on the types of bonds between carbon atoms. Understanding the general structure of hydrocarbons is essential for deducing the molecular formula once the molar mass is determined, as it helps identify possible combinations of carbon and hydrogen atoms.
Recommended video:
Guided course
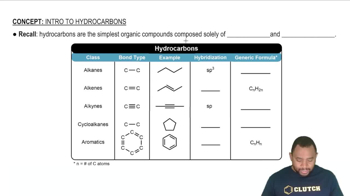
Intro To Hydrocarbons
Related Practice
Textbook Question
Textbook Question
Binary compounds of alkali metals and hydrogen react with water to liberate H2(g). The H2 from the reaction of a sample of NaH with an excess of water fills a volume of 0.490 L above the water. The temperature of the gas is 35 °C and the total pressure is 758 mmHg. Determine the mass of H2 liberated and the mass of NaH that reacted.
Textbook Question
A sample of N2O3(g) has a pressure of 0.017 atm. The temperature (in K) is doubled and the N2O3 undergoes complete decomposition to NO2(g) and NO(g). Find the total pressure of the mixture of gases assuming constant volume and no additional temperature change.
Textbook Question
A gas mixture composed of helium and argon has a density of 0.670 g/L at a 755 mmHg and 298 K. What is the composition of the mixture by volume?
