A flask at room temperature contains exactly equal amounts (in moles) of nitrogen and xenon. a. Which of the two gases exerts the greater partial pressure?

Calculate the root mean square velocity of F2, Cl2, and Br2 at 298 K.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Root Mean Square Velocity (rms velocity)
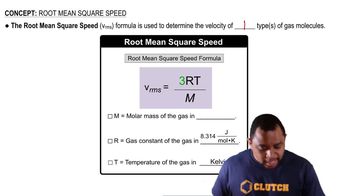
Molar Mass

Kinetic Theory of Gases

A flask at room temperature contains exactly equal amounts (in moles) of nitrogen and xenon. b. The molecules or atoms of which gas have the greater average velocity? d. If a small hole were opened in the flask, which gas effuses more quickly?
A flask at room temperature contains exactly equal amounts (in moles) of nitrogen and xenon. c. The molecules of which gas have the greater average kinetic energy?
Calculate the kinetic energy of F2, Cl2, and Br2 at 298 K.
Calculate the root mean square velocity and kinetic energy of F2, Cl2, and Br2 at 298 K. Rank these three halogens with respect to their rate of effusion.
Calculate the root mean square velocity and kinetic energy of CO, CO2, and SO3 at 298 K. Which gas has the greatest velocity? The greatest kinetic energy? The greatest effusion rate?
