Textbook Question
Calculate the molarity of each solution.
c. 32.4 mg NaCl in 122.4 mL of solution
1
views

 Verified step by step guidance
Verified step by step guidance


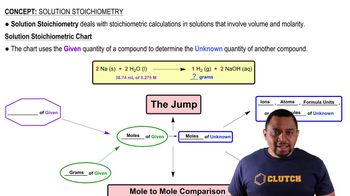
Calculate the molarity of each solution.
c. 32.4 mg NaCl in 122.4 mL of solution
Calculate the molarity of each solution. a. 0.38 mol of LiNO3 in 6.14 L of solution b. 72.8 g C2H6O in 2.34 L of solution c. 12.87 mg KI in 112.4 mL of solution
What is the molarity of NO3– in each solution? a. 0.150 M KNO3 b. 0.150 M Ca(NO3)2 c. 0.150 M Al(NO3)3
What is the molarity of Cl- in each solution? b. 0.150 M SrCl2
what is the molarity of Cl- in each solution? c. 0.100 M AlCl3
How many moles of KCl are contained in each solution? a. 0.556 L of a 2.3 M KCl solution