Textbook Question
Name each disubstituted benzene. c.

 Verified step by step guidance
Verified step by step guidance

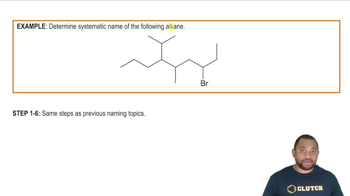

Name each disubstituted benzene. c.
Name each disubstituted benzene. b.
Name each disubstituted benzene. c.
Draw the structure for each compound. b. meta-dibromobenzene
Draw the structure for each compound. a. ethylbenzene
Draw the structure for each compound. b. 1-iodo-2-methylbenzene