Textbook Question
Draw a structure for each alkane. a. 3-ethylhexane c. 2,3-dimethylbutane

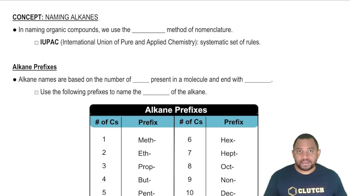
 Verified step by step guidance
Verified step by step guidance
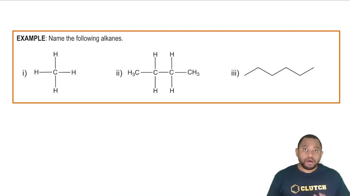

Draw a structure for each alkane. a. 3-ethylhexane c. 2,3-dimethylbutane
Draw a structure for each alkane.
b. 3-ethyl-3-methylpentane
Draw a structure for each alkane.
d. 4,7-diethyl-2,2-dimethylnonane
Draw a structure for each alkane. c. 4-ethyl-2,2-dimethylhexane
Complete and balance each hydrocarbon combustion reaction.
a. CH3CH2CH3 + O2 →
b.CH3CH2CH=CH2 +O2 →
c. CH≡CH + O2 →
Complete and balance each hydrocarbon combustion reaction. a. CH3CH2CH2CH3 + O2 → b.CH2=CHCH3 + O2 → c. CH≡CCH2CH3 + O2 →