Consider the stack of pennies in the previous problem. How much money (in dollars) would this represent? If this money were equally distributed among the world's population of 7.0 billion people, how much would each person receive? Would each person be a millionaire? A billionaire? A trillionaire?
Ch.2 - Atoms & Elements

Chapter 2, Problem 112
A pure titanium cube has an edge length of 2.78 in. How many titanium atoms does it contain? Titanium has a density of 4.50 g/cm3.
 Verified step by step guidance
Verified step by step guidance1
First, convert the edge length of the titanium cube from inches to centimeters. There are 2.54 cm in 1 inch.
Next, calculate the volume of the cube in cubic centimeters (cm3) using the formula for the volume of a cube, which is edge length cubed.
Then, calculate the mass of the titanium cube using the given density and the volume you just calculated. Density is mass divided by volume, so you can rearrange this formula to find mass = density * volume.
After that, convert the mass of the titanium cube from grams to moles using the molar mass of titanium. The molar mass of titanium is 47.87 g/mol.
Finally, calculate the number of titanium atoms in the cube using Avogadro's number, which states that there are 6.022 x 10^23 atoms in one mole of any substance.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
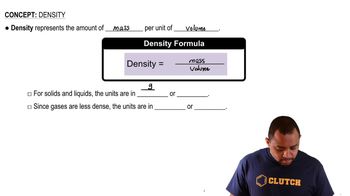
Density
Density is defined as mass per unit volume and is a crucial property of materials. In this context, the density of titanium (4.50 g/cm³) allows us to calculate the mass of the titanium cube by multiplying its volume by its density. Understanding density helps in converting between mass and volume, which is essential for determining the number of atoms in a given mass of a substance.
Recommended video:
Guided course
Density Concepts
Molar Mass
Molar mass is the mass of one mole of a substance, typically expressed in grams per mole (g/mol). For titanium, the molar mass is approximately 47.87 g/mol. This concept is vital for converting the mass of titanium obtained from the density calculation into moles, which can then be used to find the number of atoms, as one mole contains Avogadro's number (approximately 6.022 x 10²³) of atoms.
Recommended video:
Guided course

Molar Mass Concept
Volume Calculation
Volume calculation is essential for determining the space occupied by an object. For a cube, the volume can be calculated using the formula V = edge length³. In this case, converting the edge length from inches to centimeters is necessary to match the units with the density. This volume will then be used to find the mass of the titanium cube, which is a critical step in calculating the number of titanium atoms.
Recommended video:
Guided course
Constant-Volume Calorimetry
Related Practice
Textbook Question
1
views
Textbook Question
The mass of an average blueberry is 0.75 g and the mass of an automobile is 2.0×103 kg. Find the number of automobiles whose total mass is the same as 1.0 mol of blueberries.
Textbook Question
A pure copper sphere has a radius of 0.935 in. How many copper atoms does it contain? [The volume of a sphere is (4/3)πr3 and the density of copper is 8.96 g/cm3.]
Textbook Question
What is the edge length (in cm) of a titanium cube that contains 2.55 * 1024 titanium atoms? The density of titanium is 4.50 g/cm3.
2
views
