Textbook Question
Fill in the missing particles in each nuclear equation. c. 23793Np → _____ + 42He

 Verified step by step guidance
Verified step by step guidance
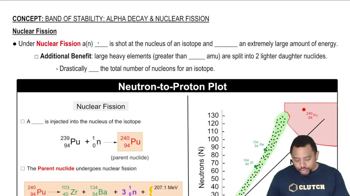

Fill in the missing particles in each nuclear equation. c. 23793Np → _____ + 42He
Fill in the missing particles in each nuclear equation. d. 7535Br → ____ + 0+1e
Determine whether or not each nuclide is likely to be stable. State your reasons. a. Mg-26 b. Ne-25 c. Co-51
Determine whether or not each nuclide is likely to be stable. State your reasons. a. Ti-48 b. Cr-63 c. Sn-102 d. Y-88
The first six elements of the first transition series have the following number of stable isotopes:
Element Number of Stable Isotopes
Sc 1
Ti 5
V 1
Cr 3
Mn 1
Fe 4
Explain why Sc, V, and Mn each have only one stable isotope while the other elements have several.
Predict a likely mode of decay for each unstable nuclide. a. Mo-109