Balance each redox reaction occurring in basic aqueous solution. a. MnO4–(aq) + Br–(aq) → MnO2(s) + BrO3–(aq)

Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the half-reaction that occurs at each electrode and the species present in each solution. Also indicate the direction of electron flow.
a. 2 Ag+(aq) + Pb(s) → 2 Ag(s) + Pb2+(aq)
b. 2 ClO2(g) + 2 I–(aq) → 2 ClO2–(aq) + I2(s)
c. O2(g) + 4 H+(aq) + 2 Zn(s) → 2 H2O(l) + 2 Zn2+(aq)
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Voltaic Cell Structure

Redox Reactions
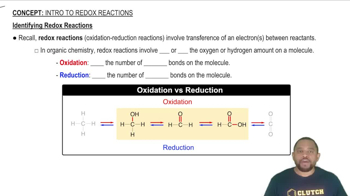
Electron Flow Direction

Balance each redox reaction occurring in basic aqueous solution. b. Ag(s) + CN–(aq) + O2(g) → Ag(CN)2–(aq)
Balance each redox reaction occurring in basic aqueous solution. c. NO2–(aq) + Al(s) → NH3(g) + AlO2–(aq)
Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the half-reaction that occurs at each electrode and the species present in each solution. Also indicate the direction of electron flow. a. Ni2+(aq) + Mg(s) → Ni(s) + Mg2+(aq)
Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the half-reaction that occurs at each electrode and the species present in each solution. Also indicate the direction of electron flow.
b. 2 H+(aq) + Fe(s) → H2(g) + Fe2+(aq)
c. 2 NO3–(aq) + 8 H+(aq) + 3 Cu(s) → 2 NO(g) + 4 H2O(l) + 3 Cu2+(aq)
Calculate the standard cell potential for each of the electro- chemical cells in Problem 43.
