Textbook Question
Consider the voltaic cell:
d. Indicate the direction of anion and cation flow in the salt bridge

 Verified step by step guidance
Verified step by step guidance

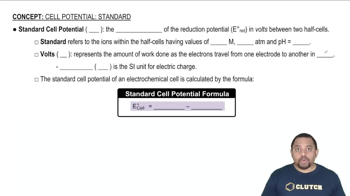
Consider the voltaic cell:
d. Indicate the direction of anion and cation flow in the salt bridge
Use line notation to represent each electrochemical cell in Problem 43.
Determine whether or not each redox reaction occurs spontaneously in the forward direction.
a. Ni(s) + Zn2+(aq) → Ni2+(aq) + Zn(s)
b. Ni(s) + Pb2+(aq) → Ni2+(aq) + Pb(s)
c. Al(s) + 3 Ag+(aq) → Al3+(aq) + 3 Ag(s)
d. Pb(s) + Mn2+(aq) → Pb2+(aq) + Mn(s)
Determine whether or not each redox reaction occurs spontaneously in the forward direction.
a. Ca2+(aq) + Zn(s) → Ca(s) + Zn2+(aq)
b. 2 Ag+(aq) + Ni(s) → 2 Ag(s) + Ni2+(aq)
c. Fe(s) + Mn2+(aq) → Fe2+(aq) + Mn(s)
d. 2 Al(s) + 3 Pb2+(aq) → 2 Al3+(aq) + 3 Pb(s)