Calculate ∆G°rxn and K for each reaction. a. The reaction of Cr2+(aq) with Cr2O72–(aq) in acid solution to form Cr3+(aq).
Ch.19 - Electrochemistry

Chapter 19, Problem 124
A metal forms the fluoride MF3. Electrolysis of the molten fluo- ride by a current of 3.86 A for 16.2 minutes deposits 1.25 g of the metal. Calculate the molar mass of the metal.
 Verified step by step guidance
Verified step by step guidance1
Convert the time from minutes to seconds by multiplying 16.2 minutes by 60 seconds per minute.
Calculate the total charge passed during electrolysis using the formula: \( Q = I \times t \), where \( I \) is the current in amperes and \( t \) is the time in seconds.
Determine the number of moles of electrons transferred using Faraday's constant (\( F = 96485 \text{ C/mol} \)). Use the formula: \( \text{moles of electrons} = \frac{Q}{F} \).
Since the metal forms the fluoride MF3, each mole of metal requires 3 moles of electrons for reduction. Calculate the moles of the metal deposited using the relation: \( \text{moles of metal} = \frac{\text{moles of electrons}}{3} \).
Calculate the molar mass of the metal using the formula: \( \text{molar mass} = \frac{\text{mass of metal}}{\text{moles of metal}} \), where the mass of the metal is given as 1.25 g.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Electrolysis
Electrolysis is a chemical process that uses electrical energy to drive a non-spontaneous reaction. In this context, it involves passing an electric current through molten fluoride to decompose it into its constituent elements, allowing for the deposition of the metal. The amount of substance deposited can be calculated using Faraday's laws of electrolysis, which relate the quantity of electric charge to the amount of substance transformed.
Recommended video:
Guided course

The Electrolytic Cell
Faraday's Laws of Electrolysis
Faraday's laws state that the amount of substance deposited during electrolysis is directly proportional to the electric charge passed through the electrolyte. The first law quantifies this relationship, while the second law relates the amount of substance to its equivalent weight. These laws are essential for calculating the molar mass of the metal in the given problem, as they allow us to connect the current, time, and mass of the deposited metal.
Recommended video:
Guided course
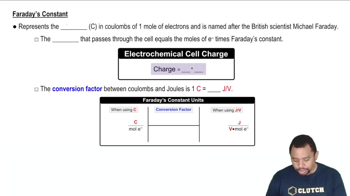
Faraday's Constant in Electrochemistry
Molar Mass Calculation
Molar mass is defined as the mass of one mole of a substance, typically expressed in grams per mole (g/mol). To calculate the molar mass of the metal in this scenario, we first determine the number of moles of metal deposited using the mass and then apply the relationship between moles, charge, and the number of electrons transferred during electrolysis. This calculation is crucial for identifying the identity of the metal based on its molar mass.
Recommended video:
Guided course
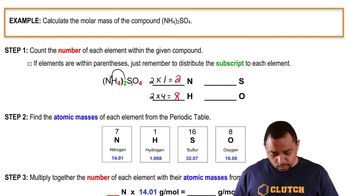
Molar Mass Calculation Example
Related Practice
Textbook Question
Textbook Question
Calculate ∆G°rxn and K for each reaction. b. The reaction of Cr3+(aq) and Cr(s) to form Cr2+(aq). [The electrode potential of Cr2+(aq) to Cr(s) is -0.91 V.]
Textbook Question
A sample of impure tin of mass 0.535 g is dissolved in strong acid to give a solution of Sn2+. The solution is then titrated with a 0.0448 M solution of NO3–, which is reduced to NO(g). The equivalence point is reached upon the addition of 0.0344 L of the NO3– solution. Find the percent by mass of tin in the original sample, assuming that it contains no other reducing agents.
Textbook Question
A current of 11.3 A is applied to 1.25 L of a solution of 0.552 M HBr converting some of the H+ to H2(g), which bubles out of solution. What is the pH of the solution after 73 minutes?
