Textbook Question
Silver can be electroplated at the cathode of an electrolysis cell by the half-reaction: Ag+(aq) + e– → Ag(s) What mass of silver would plate onto the cathode if a current of 6.8 A flowed through the cell for 72 min?

 Verified step by step guidance
Verified step by step guidance

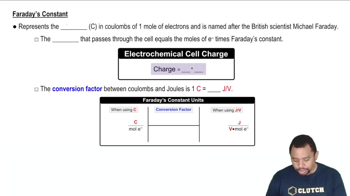

Silver can be electroplated at the cathode of an electrolysis cell by the half-reaction: Ag+(aq) + e– → Ag(s) What mass of silver would plate onto the cathode if a current of 6.8 A flowed through the cell for 72 min?
Consider the reaction shown here occurring at 25°C. Cr(s) + Cd2+(aq) → Cr2+(aq) + Cd(s) Determine E°cell, K, and ∆G°rxn for the reaction and complete the table.
[Cd2+] [Cr2+] Q Ecell 𝚫Grxn
1.00 1.00
1.00 1.00 × 10-5
1.00 × 10-5 1.00
4.18 × 10-4 1.00