Use data from Appendix IIB to calculate ΔS°rxn for each of the reactions. In each case, try to rationalize the sign of ΔS°rxn. d. N2O4(g) + 4 H2(g) → N2(g) + 4 H2O(g)
Ch.18 - Free Energy and Thermodynamics

Chapter 18, Problem 59
Methanol (CH3OH) burns in oxygen to form carbon dioxide and water. Write a balanced equation for the combustion of liquid methanol and calculate ΔH°rxn, ΔS°rxn, and ΔG°rxn at 25 °C. Is the combustion of methanol spontaneous?
 Verified step by step guidance
Verified step by step guidance1
Write the unbalanced chemical equation for the combustion of methanol: \( \text{CH}_3\text{OH} + \text{O}_2 \rightarrow \text{CO}_2 + \text{H}_2\text{O} \).
Balance the chemical equation by ensuring the number of atoms of each element is the same on both sides: \( 2\text{CH}_3\text{OH} + 3\text{O}_2 \rightarrow 2\text{CO}_2 + 4\text{H}_2\text{O} \).
Use standard enthalpies of formation to calculate \( \Delta H^\circ_{\text{rxn}} \) using the formula: \( \Delta H^\circ_{\text{rxn}} = \sum \Delta H^\circ_{\text{f,products}} - \sum \Delta H^\circ_{\text{f,reactants}} \).
Calculate \( \Delta S^\circ_{\text{rxn}} \) using standard molar entropies: \( \Delta S^\circ_{\text{rxn}} = \sum S^\circ_{\text{products}} - \sum S^\circ_{\text{reactants}} \).
Determine \( \Delta G^\circ_{\text{rxn}} \) using the Gibbs free energy equation: \( \Delta G^\circ_{\text{rxn}} = \Delta H^\circ_{\text{rxn}} - T\Delta S^\circ_{\text{rxn}} \), and assess spontaneity by checking if \( \Delta G^\circ_{\text{rxn}} < 0 \).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Combustion Reactions
Combustion reactions are exothermic processes where a substance reacts with oxygen to produce heat, light, carbon dioxide, and water. In the case of methanol (CH3OH), the balanced equation for its combustion involves the complete reaction with oxygen to yield carbon dioxide (CO2) and water (H2O). Understanding how to balance these equations is crucial for stoichiometric calculations and thermodynamic assessments.
Recommended video:
Guided course
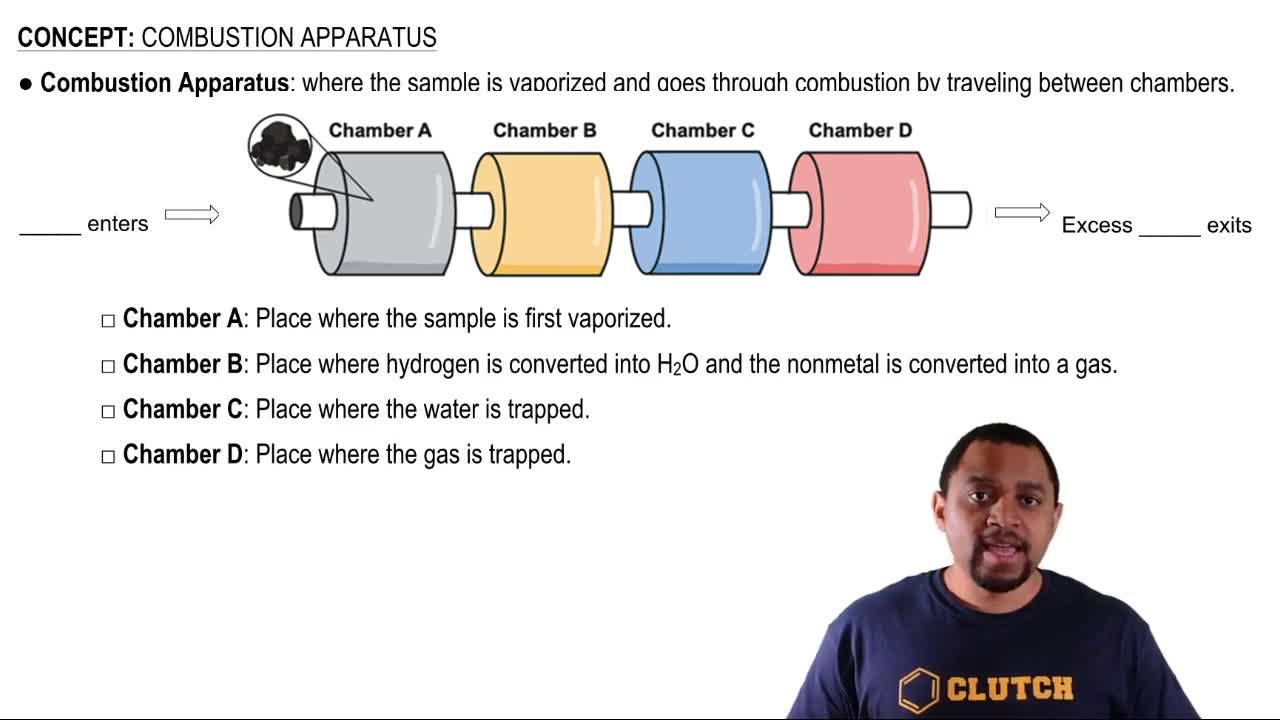
Combustion Apparatus
Thermodynamics: ΔH°, ΔS°, and ΔG°
Thermodynamics involves the study of energy changes in chemical reactions. ΔH° (enthalpy change) indicates the heat absorbed or released, ΔS° (entropy change) measures the disorder or randomness in the system, and ΔG° (Gibbs free energy change) determines the spontaneity of the reaction. A negative ΔG° suggests that the reaction is spontaneous under standard conditions, which is essential for evaluating the combustion of methanol.
Recommended video:
Guided course

First Law of Thermodynamics
Spontaneity of Reactions
The spontaneity of a reaction refers to its ability to occur without external intervention. It is determined by the sign of ΔG°; if ΔG° is negative, the reaction is spontaneous. Factors influencing spontaneity include enthalpy and entropy changes, where a highly exothermic reaction with a significant increase in entropy is likely to be spontaneous, as is the case with the combustion of methanol.
Recommended video:
Guided course
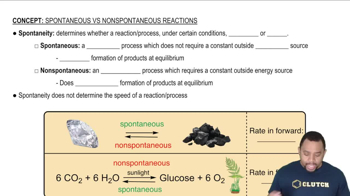
Spontaneity of Processes
Related Practice
Textbook Question
Textbook Question
Find ΔS° for the formation of CH2Cl2(g) from its gaseous elements in their standard states. Rationalize the sign of ΔS°.
Textbook Question
In photosynthesis, plants form glucose (C6H12O6) and oxygen from carbon dioxide and water. Write a balanced equation for photosynthesis and calculate ΔH°rxn, ΔS°rxn, and ΔG°rxn at 25 °C. Is photosynthesis spontaneous?
1
views
Textbook Question
For each reaction, calculate ΔH°rxn, ΔS°rxn, and ΔG°rxn at 25 °C and state whether or not the reaction is spontaneous. If the reaction is not spontaneous, would a change in temperature make it spontaneous? If so, should the temperature be raised or lowered from 25 °C? a. N2O4(g) → 2 NO2(g)
