Predict the conditions (high temperature, low temperature, all temperatures, or no temperatures) under which each reaction is spontaneous. a. H2O(g) → H2O(l) b. CO2(s) → CO2(g) c. H2(g) → 2 H(g) d. 2 NO2(g) → 2 NO(g) + O2(g) (endothermic)

For each pair of substances, choose the one that you expect to have the higher standard molar entropy (S°) at 25 °C. Explain your choices. a. NaNO3(s); NaNO3(aq) b. CH4(g); CH3CH3(g) c. Br2(l); Br2(g) d. Br2(g); F2(g) e. PCl3(g); PCl5(g) f. CH3CH2CH2CH3(g); SO2(g)
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Standard Molar Entropy (S°)
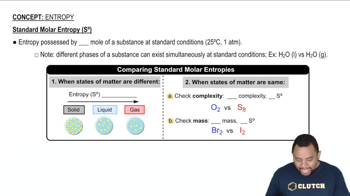
Phase Changes and Entropy

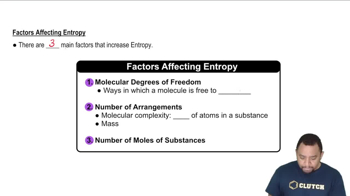
Molecular Complexity and Entropy
How does the molar entropy of a substance change with increasing temperature?
For each pair of substances, choose the one that you expect to have the higher standard molar entropy (S°) at 25 °C. Explain your choices. a. CO(g); CO2(g) b. CH3OH(l); CH3OH(g) c. Ar(g); CO2(g) d. CH4(g); SiH4(g) e. NO2(g); CH3CH2CH3(g) f. NaBr(s); NaBr(aq)
Rank each set of substances in order of increasing standard molar entropy (S°). Explain your reasoning. a. NH3(g); Ne(g); SO2(g); CH3CH2OH(g); He(g) c. CH4(g); CF4(g); CCl4(g)
Rank each set of substances in order of increasing standard molar entropy (S°). Explain your reasoning. b. H2O(s); H2O(l); H2O(g)
Rank each set of substances in order of increasing standard molar entropy (S°). Explain your reasoning. a. I2(g); F2(g); Br2(g); Cl2(g) b. H2O(g); H2O2(g); H2S(g) c. C(s, graphite); C(s, diamond); C(s, amorphous)
