A buffer contains significant amounts of acetic acid and sodium acetate. Write equations showing how this buffer neutralizes added acid and added base.
Ch.17 - Aqueous Ionic Equilibrium

Chapter 17, Problem 38
Use the Henderson–Hasselbalch equation to calculate the pH of each solution in Problem 30.
 Verified step by step guidance
Verified step by step guidance1
Identify the components of the buffer solution: the weak acid (HA) and its conjugate base (A^-).
Determine the concentrations of the weak acid [HA] and its conjugate base [A^-] in the solution.
Find the pKa of the weak acid. This can often be found in a table of acid dissociation constants.
Use the Henderson–Hasselbalch equation: \( \text{pH} = \text{pKa} + \log \left( \frac{[A^-]}{[HA]} \right) \).
Substitute the values of pKa, [A^-], and [HA] into the equation to calculate the pH.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Henderson-Hasselbalch Equation
The Henderson-Hasselbalch equation is a mathematical formula used to calculate the pH of a buffer solution. It relates the pH of the solution to the pKa of the acid and the ratio of the concentrations of the conjugate base to the acid. The equation is expressed as pH = pKa + log([A-]/[HA]), where [A-] is the concentration of the base and [HA] is the concentration of the acid.
Recommended video:
Guided course
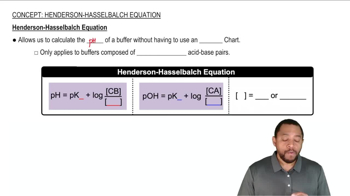
Henderson-Hasselbalch Equation
Buffer Solutions
Buffer solutions are mixtures that can resist changes in pH when small amounts of acid or base are added. They typically consist of a weak acid and its conjugate base or a weak base and its conjugate acid. Understanding how buffers work is essential for applying the Henderson-Hasselbalch equation, as it helps in determining how the pH will change in response to the addition of acids or bases.
Recommended video:
Guided course
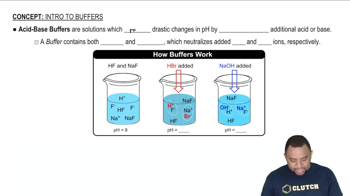
Buffer Solutions
pKa and Acid-Base Equilibrium
The pKa is a measure of the strength of an acid in solution, defined as the negative logarithm of the acid dissociation constant (Ka). It indicates the pH at which half of the acid is dissociated into its conjugate base. Knowing the pKa of the acid involved in the buffer system is crucial for using the Henderson-Hasselbalch equation effectively, as it helps predict the pH based on the concentrations of the acid and its conjugate base.
Recommended video:
Guided course
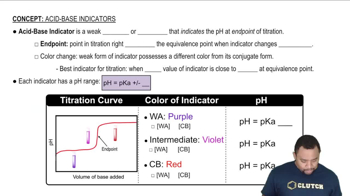
Acid-Base Indicators
Related Practice
Textbook Question
1
views
Textbook Question
A buffer contains significant amounts of ammonia and ammonium chloride. Write equations showing how this buffer neutralizes added acid and added base.
1
views
Textbook Question
Use the Henderson–Hasselbalch equation to calculate the pH of each solution in Problem 29.
Textbook Question
Use the Henderson–Hasselbalch equation to calculate the pH of each solution. c. a solution that contains 10.0 g of HC2H3O2 and 10.0 g of NaC2H3O2 in 150.0 mL of solution
1
views
Textbook Question
Calculate the pH of the solution that results from each mixture. a. 50.0 mL of 0.15 M HCHO2 with 75.0 mL of 0.13 M NaCHO2
1
views
Textbook Question
Calculate the pH of the solution that results from each mixture. b. 125.0 mL of 0.10 M NH3 with 250.0 mL of 0.10 M NH4Cl
