Use the given molar solubilities in pure water to calculate Ksp for each compound. a. MX; molar solubility = 3.27⨉10-11 M
Ch.17 - Aqueous Ionic Equilibrium

Chapter 17, Problem 90b,c
Use the given molar solubilities in pure water to calculate Ksp for each compound. b. Ag2SO3; molar solubility = 1.55⨉10-5 M c. Pd(SCN)2; molar solubility = 2.22⨉10-8 M
 Verified step by step guidance
Verified step by step guidance1
Identify the dissolution equation for Ag_2SO_3: Ag_2SO_3(s) \rightleftharpoons 2Ag^+(aq) + SO_3^{2-}(aq).
Determine the relationship between molar solubility and ion concentrations: If the molar solubility of Ag_2SO_3 is s, then [Ag^+] = 2s and [SO_3^{2-}] = s.
Substitute the given molar solubility into the expressions for ion concentrations: [Ag^+] = 2(1.55 \times 10^{-5}) M and [SO_3^{2-}] = 1.55 \times 10^{-5} M.
Write the expression for the solubility product constant, K_{sp}: K_{sp} = [Ag^+]^2[SO_3^{2-}].
Substitute the ion concentrations into the K_{sp} expression and simplify: K_{sp} = (2 \times 1.55 \times 10^{-5})^2 \times (1.55 \times 10^{-5}).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Molar Solubility
Molar solubility is the number of moles of a solute that can dissolve in one liter of solution at a given temperature. It is a crucial measure in determining how much of a compound can be present in a saturated solution. In this context, the molar solubility of Ag₂SO₃ is given as 1.55×10⁻⁵ M, which indicates the maximum concentration of the compound that can dissolve in water.
Recommended video:
Guided course
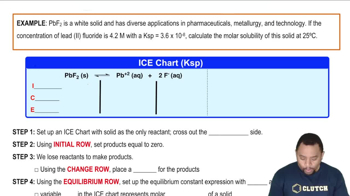
Molar Solubility Example
Solubility Product Constant (Ksp)
The solubility product constant, Ksp, is an equilibrium constant that applies to the solubility of ionic compounds. It is defined as the product of the molar concentrations of the ions, each raised to the power of their coefficients in the balanced equation. For Ag₂SO₃, Ksp can be calculated using the molar solubility to find the concentrations of Ag⁺ and SO₃²⁻ ions in a saturated solution.
Recommended video:
Guided course

Solubility Product Constant
Dissociation of Ionic Compounds
When ionic compounds dissolve in water, they dissociate into their constituent ions. For Ag₂SO₃, the dissociation can be represented as Ag₂SO₃(s) ⇌ 2Ag⁺(aq) + SO₃²⁻(aq). Understanding this dissociation is essential for calculating Ksp, as it allows us to relate the molar solubility to the concentrations of the ions produced in solution.
Recommended video:
Guided course

Ionic Compounds Naming
Related Practice
Textbook Question
Textbook Question
Use the given molar solubilities in pure water to calculate Ksp for each compound. b. PbF2; molar solubility = 5.63⨉10-3 M c. MgF2; molar solubility = 2.65⨉10-4 M
Textbook Question
Use the given molar solubilities in pure water to calculate Ksp for each compound. a. BaCrO4; molar solubility = 1.08⨉10-5 M
Textbook Question
Two compounds with general formulas AX and AX2 have Ksp = 1.5⨉10-5. Which of the two compounds has the higher molar solubility?
Textbook Question
Consider the compounds with the generic formulas listed and their corresponding molar solubilities in pure water. Which compound has the smallest value of Ksp? a. AX; molar solubility = 1.35⨉10-4 M b. AX2; molar solubility = 2.25⨉10-4 M c. A2X; molar solubility = 1.75⨉10-4 M
