Textbook Question
A solution is made 1.1⨉10-3 M in Zn(NO3)2 and 0.150 M in NH3. After the solution reaches equilibrium, what concentration of Zn2+(aq) remains?

 Verified step by step guidance
Verified step by step guidance

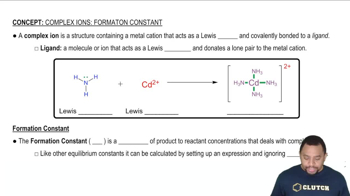

A solution is made 1.1⨉10-3 M in Zn(NO3)2 and 0.150 M in NH3. After the solution reaches equilibrium, what concentration of Zn2+(aq) remains?
Use the appropriate values of Ksp and Kf to find the equilibrium constant for the reaction. FeS(s) + 6 CN-(aq) ⇌ Fe(CN)64-(aq) + S2-(aq)