Two 25.0-mL samples, one 0.100 M HCl and the other 0.100 M HF, are titrated with 0.200 M KOH. b. Is the pH at the equivalence point for each titration acidic, basic, or neutral?
Ch.17 - Aqueous Ionic Equilibrium

Chapter 17, Problem 62d
Two 25.0-mL samples, one 0.100 M HCl and the other 0.100 M HF, are titrated with 0.200 M KOH.
d. Sketch each titration curve.
 Verified step by step guidance
Verified step by step guidance1
Start by understanding the nature of the acids involved: HCl is a strong acid, while HF is a weak acid. This will affect the shape of the titration curves.
For the titration of HCl with KOH, recognize that the equivalence point will occur when the moles of HCl equal the moles of KOH added. Since both are strong electrolytes, the pH at the equivalence point will be neutral (pH = 7).
For the titration of HF with KOH, note that HF is a weak acid. The equivalence point will occur when the moles of HF equal the moles of KOH added, but the pH at the equivalence point will be greater than 7 due to the formation of the weak base F-.
Sketch the titration curve for HCl: Start with a low pH, increase steadily as KOH is added, and reach a sharp rise at the equivalence point, leveling off at pH 7.
Sketch the titration curve for HF: Start with a higher initial pH than HCl, increase gradually, and at the equivalence point, the curve will rise more gradually and level off at a pH greater than 7.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
5mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Titration Curves
Titration curves graphically represent the change in pH of a solution as a titrant is added. The shape of the curve depends on the strength of the acid and base involved. Strong acids, like HCl, show a sharp increase in pH at the equivalence point, while weak acids, like HF, exhibit a more gradual change due to their incomplete dissociation.
Recommended video:
Guided course
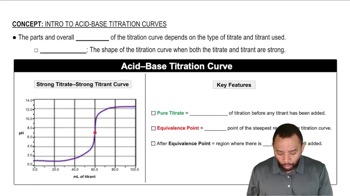
Acid-Base Titration Curves
Strong vs. Weak Acids
Strong acids, such as HCl, completely dissociate in water, resulting in a high concentration of hydrogen ions (H+). In contrast, weak acids like HF only partially dissociate, leading to a lower concentration of H+. This difference affects the pH at various points in the titration and the overall shape of the titration curve.
Recommended video:
Guided course
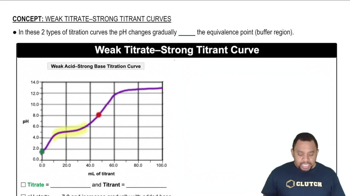
Weak Acid-Strong Base Titration Curve
Equivalence Point
The equivalence point in a titration is reached when the amount of titrant added is stoichiometrically equivalent to the amount of substance in the sample. At this point, the pH of the solution changes dramatically, especially in strong acid-strong base titrations. For weak acids, the pH at the equivalence point will be higher than 7 due to the formation of the conjugate base.
Recommended video:
Guided course
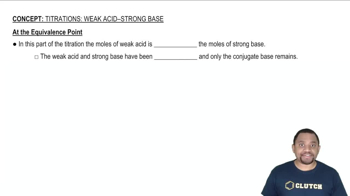
At the Equivalence Point
Related Practice
Textbook Question
Textbook Question
Two 25.0-mL samples, one 0.100 M HCl and the other 0.100 M HF, are titrated with 0.200 M KOH. c. Which titration curve has the lower initial pH?
Textbook Question
Two 20.0-mL samples, one 0.200 M KOH and the other 0.200 M CH3NH2, are titrated with 0.100 M HI. a. What is the volume of added acid at the equivalence point for each titration?
Textbook Question
Two 20.0-mL samples, one 0.200 M KOH and the other 0.200 M CH3NH2, are titrated with 0.100 M HI. b. Is the pH at the equivalence point for each titration acidic, basic, or neutral?
Textbook Question
Two 20.0-mL samples, one 0.200 M KOH and the other 0.200 M CH3NH2, are titrated with 0.100 M HI. c. Which titration curve has the lower initial pH?
