A formic acid solution has a pH of 3.25. Which of these substances will raise the pH of the solution upon addition? Explain your answer. a. HCl b. NaBr c. NaCHO2 d. KCl
Ch.17 - Aqueous Ionic Equilibrium

Chapter 17, Problem 30
Solve an equilibrium problem (using an ICE table) to calculate the pH of each solution. a. a solution that is 0.195 M in HC2H3O2 and 0.125 M in KC2H3O2 b. a solution that is 0.255 M in CH3NH2 and 0.135 M in CH3NH3Br
 Verified step by step guidance
Verified step by step guidance1
Identify the type of solution: Both solutions are buffer solutions. Solution (a) is an acetic acid (HC2H3O2) and acetate ion (C2H3O2-) buffer, while solution (b) is a methylamine (CH3NH2) and methylammonium ion (CH3NH3+) buffer.
Use the Henderson-Hasselbalch equation for buffer solutions: \( \text{pH} = \text{pK}_a + \log \left( \frac{[\text{A}^-]}{[\text{HA}]} \right) \) for acidic buffers and \( \text{pOH} = \text{pK}_b + \log \left( \frac{[\text{B}]}{[\text{BH}^+]} \right) \) for basic buffers.
For solution (a), find the \( \text{pK}_a \) of acetic acid (HC2H3O2) using a reference table. Then, substitute \([\text{A}^-] = 0.125 \text{ M}\) and \([\text{HA}] = 0.195 \text{ M}\) into the Henderson-Hasselbalch equation to find the pH.
For solution (b), find the \( \text{pK}_b \) of methylamine (CH3NH2) using a reference table. Then, substitute \([\text{B}] = 0.255 \text{ M}\) and \([\text{BH}^+] = 0.135 \text{ M}\) into the Henderson-Hasselbalch equation to find the pOH.
Convert the pOH to pH for solution (b) using the relation \( \text{pH} = 14 - \text{pOH} \).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Equilibrium and ICE Tables
An ICE table (Initial, Change, Equilibrium) is a tool used to organize the concentrations of reactants and products in a chemical equilibrium problem. It helps in calculating the changes in concentration that occur as the system reaches equilibrium. Understanding how to set up and interpret an ICE table is crucial for solving equilibrium problems, including those involving weak acids and bases.
Recommended video:
Guided course

ICE Charts and Equilibrium Amount
pH and Acid-Base Chemistry
pH is a measure of the acidity or basicity of a solution, defined as the negative logarithm of the hydrogen ion concentration. In the context of weak acids and their conjugate bases, the pH can be calculated using the Henderson-Hasselbalch equation, which relates the pH of a buffer solution to the concentration of the acid and its conjugate base. This concept is essential for determining the pH of solutions containing weak acids and their salts.
Recommended video:
Guided course
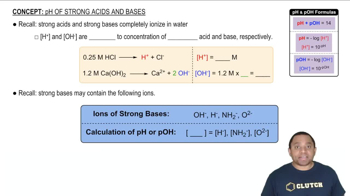
pH of Strong Acids and Bases
Buffer Solutions
A buffer solution is a system that resists changes in pH upon the addition of small amounts of acid or base. It typically consists of a weak acid and its conjugate base or a weak base and its conjugate acid. Understanding how buffers work is important for calculating the pH of solutions that contain both a weak acid and its salt, as seen in the given problem.
Recommended video:
Guided course
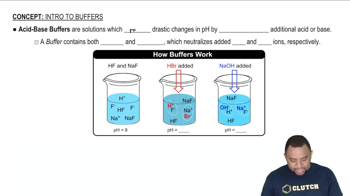
Buffer Solutions
Related Practice
Textbook Question
Textbook Question
Solve an equilibrium problem (using an ICE table) to calculate the pH of each solution. a. a solution that is 0.20 M in HCHO2 and 0.15 M in NaCHO2
Textbook Question
Solve an equilibrium problem (using an ICE table) to calculate the pH of each solution. b. a solution that is 0.16 M in NH3 and 0.22 M in NH4Cl
Textbook Question
Calculate the percent ionization of a 0.15 M benzoic acid solution in pure water and in a solution containing 0.10 M sodium benzoate. Why does the percent ionization differ significantly in the two solutions?
Textbook Question
Solve an equilibrium problem (using an ICE table) to calculate the pH of each solution. a. 0.15 M HF
