Textbook Question
A buffer contains significant amounts of acetic acid and sodium acetate. Write equations showing how this buffer neutralizes added acid and added base.
1
views

 Verified step by step guidance
Verified step by step guidance
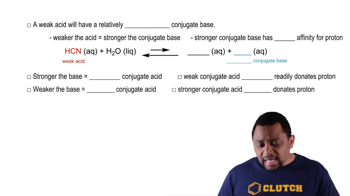

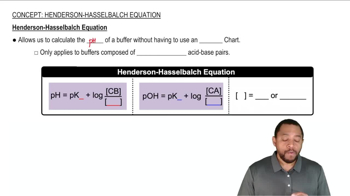
A buffer contains significant amounts of acetic acid and sodium acetate. Write equations showing how this buffer neutralizes added acid and added base.
Solve an equilibrium problem (using an ICE table) to calculate the pH of each solution. a. 0.15 M HF
Solve an equilibrium problem (using an ICE table) to calculate the pH of each solution. b. 0.15 M NaF
Solve an equilibrium problem (using an ICE table) to calculate the pH of each solution. a. 0.18 M CH3NH2 b. 0.18 M CH3NH3Cl c. a mixture that is 0.18 M in CH3NH2 and 0.18 M in CH3NH3Cl
A buffer contains significant amounts of ammonia and ammonium chloride. Write equations showing how this buffer neutralizes added acid and added base.