Textbook Question
Write balanced equations and expressions for Ksp for the dissolution of each ionic compound. b. PbBr2

 Verified step by step guidance
Verified step by step guidance

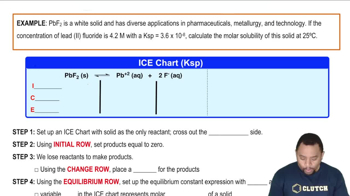

Write balanced equations and expressions for Ksp for the dissolution of each ionic compound. b. PbBr2
Write balanced equations and expressions for Ksp for the dissolution of each ionic compound. c. Ag2CrO4
Write balanced equations and expressions for Ksp for the dissolution of each ionic compound. a. CaCO3 b. PbCl2 c. AgI
Refer to the Ksp values in Table 17.2 to calculate the molar solubility of each compound in pure water. b. Mg(OH)2 c. CaF2
Refer to the Ksp values in Table 17.2 to calculate the molar solubility of each compound in pure water. a. MX (Ksp = 1.27⨉10-36) b. Ag2CrO4 c. Ca(OH)2
Use the given molar solubilities in pure water to calculate Ksp for each compound. a. MX; molar solubility = 3.27⨉10-11 M